The quart of Sunnyside Xylol (xylenes) arrived yesterday. The only hazard labeling was the blue ORM-D shown in the packaging picture. No signature was necessary for delivery.
I was pouring the contents of the can into a reagent bottle and noticed a grey tint, and then due to there being more than expected in the can I poured the remainder into a smaller bottle, and found the grey mass impurity, you can see globs of it in the picture. It doesn't dissolve in the xylene, and it seems to have been completely removed by simply filtering through 2.5 micron lab filter paper. There are other sources of xylene, so I won't go on further about it.
https://en.wikipedia.org/wiki/Xylene
"Xylene is used as a solvent. In this application, with a mixture of isomers, it is often referred to as xylenes or xylol. Solvent xylene often contains a small percentage of ethylbenzene. Like the individual isomers, the mixture is colorless, sweet-smelling, and highly flammable.
Xylene is flammable but of modest acute toxicity, with LD50 ranges from 200 to 5000 mg/kg for animals. Oral LD50 for rats is 4300 mg/kg. The principal mechanism of detoxification is oxidation to methylbenzoic acid and hydroxylation to hydroxylene.
The main effect of inhaling xylene vapor is depression of the central nervous system (CNS), with symptoms such as headache, dizziness, nausea and vomiting. At an exposure of 100 ppm, one may experience nausea or a headache. At an exposure between 200-500 ppm, symptoms can include feeling "high", dizziness, weakness, irritability, vomiting, slowed reaction time.
The side effects of exposure to low concentrations of xylene (< 200 ppm) are reversible and do not cause permanent damage.
One report contained data that shows that long-term exposure to low levels of xylene led to a decrease in balance, coordination, and reaction times in participants. These are examples of how it affects the CNS.
Long-term exposure may lead to headaches, irritability, depression, insomnia, agitation, extreme tiredness, tremors, impaired concentration and short-term memory. This condition is sometimes generally referred to as "organic solvent syndrome". Unfortunately, there is very little information available that isolates xylene from other solvent exposures in the examination of these effects.
Xylene is also a skin irritant and strips the skin of its oils, making it more permeable to other chemicals.
To reduce the risk of developing health problems from occupational exposure, one must wear gloves and a mask to prevent high concentrations of inhalation and skin irritation from Xylene."
It is extremely non-polar, compare at the list my cache of solvents arranged as to polarity,
https://www.icmag.com/ic/showpost.php?p=7933632&postcount=162
The problem with it is it has a strong odor, reminds me of the odor of felt tip markers from decades ago, you can sniff it and get dizzy. On the other hand, get some on your fingers, and at the point of complete evaporation, the odor completely disappears, happens like magic, so it might be purgeable, and for sure you'll know if you got it all out.
The ethyl formate is scheduled for delivery today.
The quart of Klean-Strip Xylol/Xylene arrived. Only the Consumer Commodity ORM-D mention on the label, no signature required, it was left at the door. The plastic cap shown in the pictures easily lifts to open, there was no inner seal. Same strong odor as Sunnyside's.



































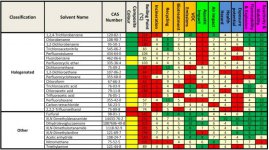


 Anyone ever played with Paris III software? "Program Assisting Replacement of Industrial Solvents" It can be downloaded for free from EPA, but needs to be unpacked and tweaked for proper operation on your particular OS. it is basically open source
Anyone ever played with Paris III software? "Program Assisting Replacement of Industrial Solvents" It can be downloaded for free from EPA, but needs to be unpacked and tweaked for proper operation on your particular OS. it is basically open source 







