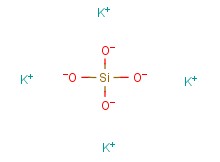
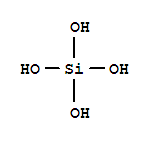
What is your definition for "siliconized"?
Let's start with this definition: The process which amorphous and silicic acid found in the soil are converted to "soluble silica"...and ultimately converted to "Plant Available Silica".
Of course all Si components are not equal, rice hull ash, diatomaceous earth, Wollastonite, and various chemical/synthetic silicates (calcium silicate, potassium silicate, etc), each have different paths/processes to become "soluble Si"--before the Si becomes "PAS".
And then we have the $/lb analysis. Why pay $6/lb for a chemical/synthetic SiO2...when $0.60/lb OMRI/Organic/natural product delivers more? You know, the old fashion "cost benefit analysis"...but now we are in the weeds and beyond the scope of this particular thread.