Aveng & Only,
Let me respond with some tables & graphs.
First let's post the Table Aveng is referring to from the Silicon Conference. I have two comments about that Table--
1. The Riice industry requires slag sources of Si (which are not "organic") to be at least 20% soluble--but also contain industrial contaminants including traces of heavy metals.
2. Notice Potassium Silicate is almost last (3rd from the bottom) and was bested by the lower grade DE tested (FSF is 41.6% Si).

And in that same Conference Report Aveng referred to, on page 152 there is also a study on AgriPower Enhanced DE (sourced from fresh water diatoms and finely milled, just like FSF). Instead of performing one type of extraction test (like the above table), they did three (alkaline, acid and neutral extraction techniques) and notice that slags did not do so well in these group of tests.
First those graphs--
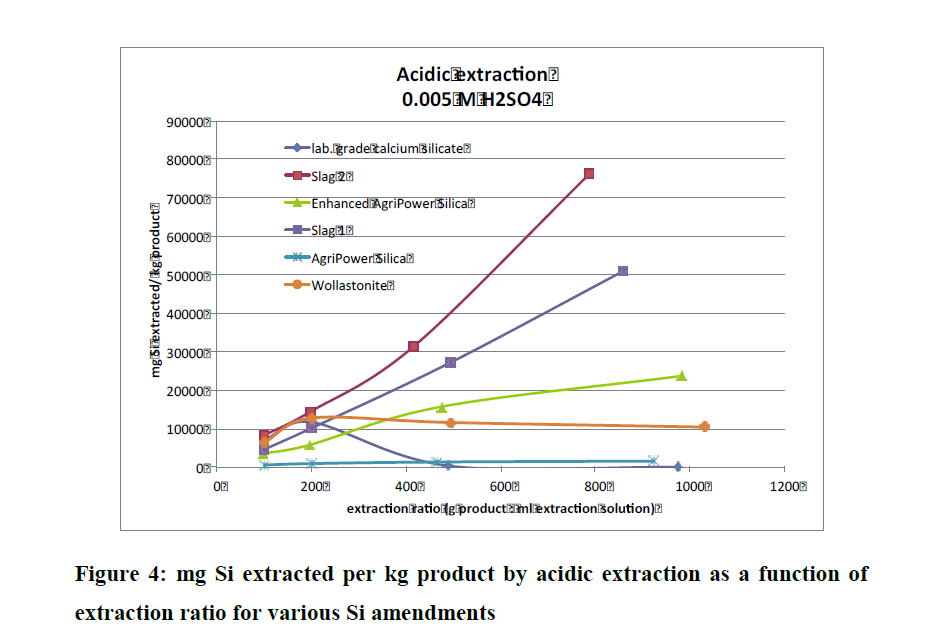
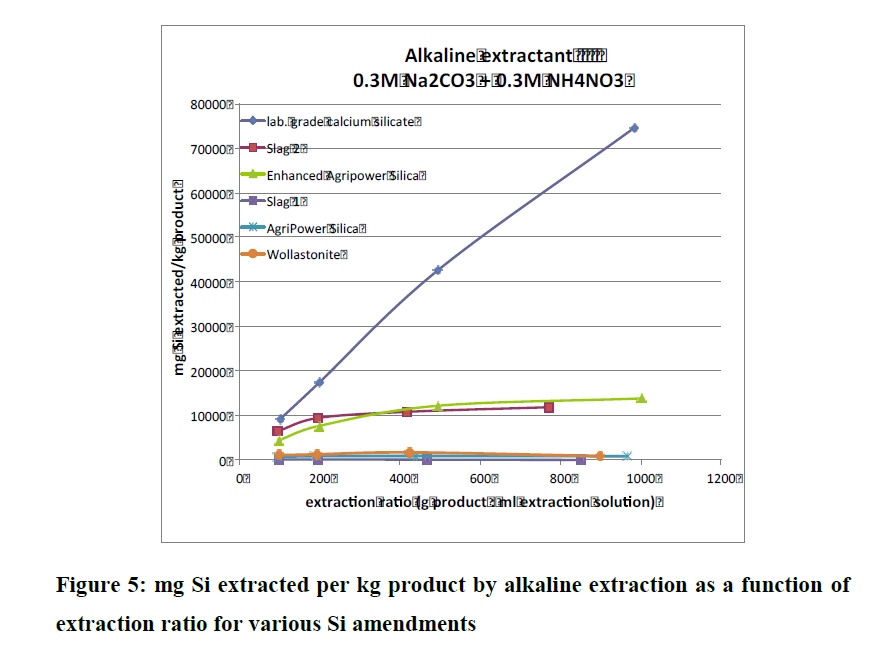
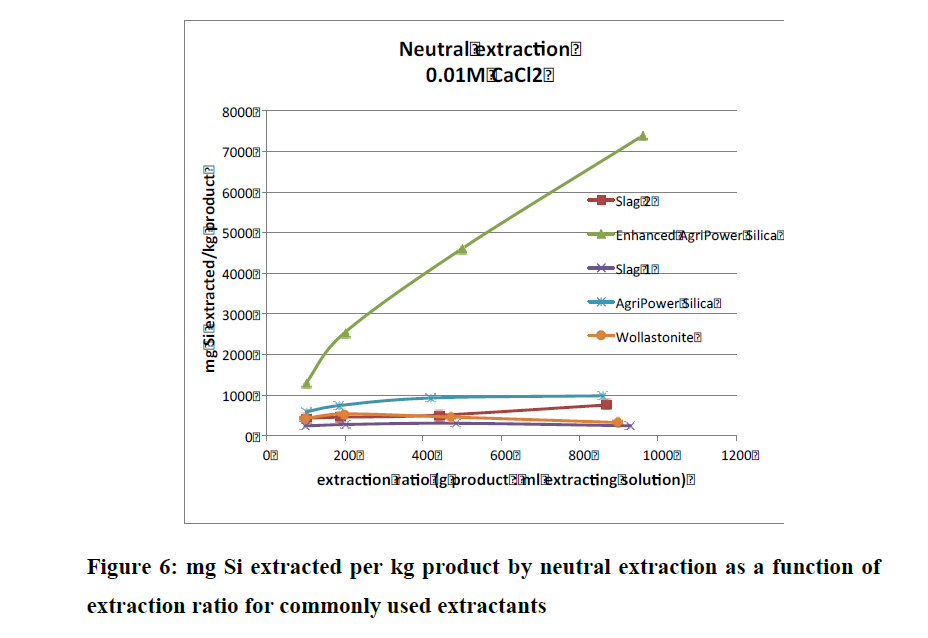
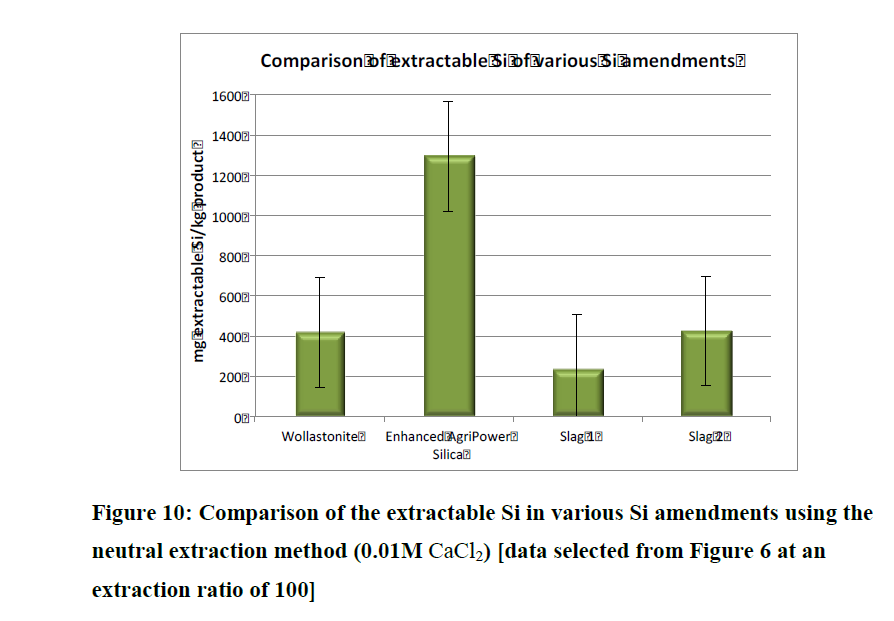
And that paper's conclusion--
The inclusion of AgriPower Silica into the nutrition program of several crops as a base soil conditioner ahead of planting delivered significant improvements in crop productivity and the gross margin per hectare grown. This was attributed to diatomaceous earth‘s ability to increase nutrient retention and plant uptake, improve moisture retention and deliver plant available silicon.
NPK (Nitrogen, Phosphorous, Potassium) based fertilisers are often considered a necessary part of intensive crop cultivation to improve crop production. Problematically, these fertilisers are a major source of water pollution due to Australian soils‘ susceptibility to leaching. The initial results presented in this paper support the argument that AgriPower Silica could improve the soil retention and plant uptake of these key nutrients, indicating the potential for AgriPower Silica to displace a significant portion of NPK fertilisers. A reduced requirement of urea and phosphate inputs would provide an economic benefit and reduce the environmental impact of these fertilisers. AN improved crop yield due to the application of AgriPower Silica similarly provides an economic benefit.
Calcium Silicate slag as an industrial by-product is a proven Si-rich amendment, however, it carries the risk of polluting soils and natural waters. An Enhanced AgriPower Silica was developed based on the natural AgriPower Silica. This product is free of the contaminants typically found in slags and delivers a significant amount of plant available silicon in all the four soils tested. The level of Si in the most Si-deficient soils was increased by 120% and therefore stands to benefit significantly from the Enhanced AgriPower Silica.
Let me respond with some tables & graphs.
First let's post the Table Aveng is referring to from the Silicon Conference. I have two comments about that Table--
1. The Riice industry requires slag sources of Si (which are not "organic") to be at least 20% soluble--but also contain industrial contaminants including traces of heavy metals.
2. Notice Potassium Silicate is almost last (3rd from the bottom) and was bested by the lower grade DE tested (FSF is 41.6% Si).
And in that same Conference Report Aveng referred to, on page 152 there is also a study on AgriPower Enhanced DE (sourced from fresh water diatoms and finely milled, just like FSF). Instead of performing one type of extraction test (like the above table), they did three (alkaline, acid and neutral extraction techniques) and notice that slags did not do so well in these group of tests.
First those graphs--
And that paper's conclusion--
The inclusion of AgriPower Silica into the nutrition program of several crops as a base soil conditioner ahead of planting delivered significant improvements in crop productivity and the gross margin per hectare grown. This was attributed to diatomaceous earth‘s ability to increase nutrient retention and plant uptake, improve moisture retention and deliver plant available silicon.
NPK (Nitrogen, Phosphorous, Potassium) based fertilisers are often considered a necessary part of intensive crop cultivation to improve crop production. Problematically, these fertilisers are a major source of water pollution due to Australian soils‘ susceptibility to leaching. The initial results presented in this paper support the argument that AgriPower Silica could improve the soil retention and plant uptake of these key nutrients, indicating the potential for AgriPower Silica to displace a significant portion of NPK fertilisers. A reduced requirement of urea and phosphate inputs would provide an economic benefit and reduce the environmental impact of these fertilisers. AN improved crop yield due to the application of AgriPower Silica similarly provides an economic benefit.
Calcium Silicate slag as an industrial by-product is a proven Si-rich amendment, however, it carries the risk of polluting soils and natural waters. An Enhanced AgriPower Silica was developed based on the natural AgriPower Silica. This product is free of the contaminants typically found in slags and delivers a significant amount of plant available silicon in all the four soils tested. The level of Si in the most Si-deficient soils was increased by 120% and therefore stands to benefit significantly from the Enhanced AgriPower Silica.






