-
Happy Birthday ICMag! Been 20 years since Gypsy Nirvana created the forum! We are celebrating with a 4/20 Giveaway and by launching a new Patreon tier called "420club". You can read more here.
-
Important notice: ICMag's T.O.U. has been updated. Please review it here. For your convenience, it is also available in the main forum menu, under 'Quick Links"!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Spurr's groundbreaking fert. mixes and methods (YouTube screen-cast and web site!)
- Thread starter spurr
- Start date
This is great thanks Spurr. I too am a jean us I have a triple digit IQ as well. It's zero zero nine.
@ all,
Thanks for the kind words. I'm glad you find this info interesting and hopefully you have success putting it into practice. As I wrote on page 1, I know it's a lot to take it at one time, so maybe take your time and watch the video a couple of times(?). I know once people understand the underlying principles the rest is cake. It's really very easy to use my methodology, but it does require a few extra steps ... thankfully those steps are easy as pie via use of the three calculators to do the hard math for us
Thanks for the kind words. I'm glad you find this info interesting and hopefully you have success putting it into practice. As I wrote on page 1, I know it's a lot to take it at one time, so maybe take your time and watch the video a couple of times(?). I know once people understand the underlying principles the rest is cake. It's really very easy to use my methodology, but it does require a few extra steps ... thankfully those steps are easy as pie via use of the three calculators to do the hard math for us
This is great thanks Spurr. I too am a jean us I have a triple digit IQ as well. It's zero zero nine.
Ha. Well, considering 100 is the average IQ of most people, I think you're a little below average
This is a lot to take in lol I have homework to do.
There's a gold star waiting for you once you get an A!
There's a gold star waiting for you once you get an A!
Alright man by next year (January) I will finally have a tds meter and order everything I need. I will figure this out eventually. I know it will be worth it.
but should I stick with perlite or go with sunshine mix? With sunshine mix, do you water with plain water every other time? obviously I would use ro water and add all the different acids and sillica, but not add any nutrients? as long as the ppm and ph is good?
I know these questions have to be getting old man but when you look around and read, everyone has a different answer. I just have started to trust yours more than others.
Ingenious on using the Bru'n water to find out alkalinity. I always wonder if brewing and growing could be combine...now I have my answer
Thanks. However, I didn't try to find a beer brewing application (such as Bru'n Water) on purpose, I would have if it had dawned on me to do so; I just got lucky. I spent about a week searching the Internet for an easy to use citric acid alkalinity calculator on my Uni's databases, Google, Ixquick and DuckDuckGo search engines, to no avail. It wasn't until I used Bing search engine that I found Bru'n Water ... and I am so very glad I did find Bru'n Water. The main problem was my choice of search terms.
Before I found Bru'n Water I started getting pretty frustrated, and I even contacted UNH to ask one of the authors of Alk Calk if he would expand it to include citric acid (it already does the math for nitric acid, sulfuric acid and phosphoric acid). Lets just say I got a cool response (and not the good "cool"). However, once I told him I work in the field of plant physiology on medical cannabis* he really got unfriendly (and he's boarded by medical cannabis states in Main, Vermont and soon Mass!).
* When I e-mail or call academics at a University, College or corporation, I tell them I am a plant physiologist and consultant working with medical cannabis. And 99.9% of the time they are interested and end up asking me about cannabis and medical cannabis application, etc. The funny part is, more times than not I end up teaching them (about cannabis), not the other way around  ... cannabis is great
... cannabis is great 
I can do the math to find effects from citric acid, as is used by Bru'n Water. However, it's a major PITA (I'm not keen on lots of math) and I doubt more than a handful of people would have been able/willing to use my methodology (had i not found Bru'n Water). That is why I felt I had to find an automated calculator for citric acid, and thankfully I did find such a calculator.
I am also going to check out "EZ Water Calculator" (http://www.ezwatercalculator.com/), it too is free, however, Bru'n Water does exactly what is needed so I am satisfied at this time. However, I may hack Bru'n Water to provide only citric acid calculations. I plan to contact the Bru'n Water author and ask him if he would be willing to do so, or if he would mind if I did.
I am thinking about making a simple spreadsheet that includes math for citric acid, phosphoric acid, sulfuric acid and nitric acid. Basically, I want to merge Bru'n Water and UNH Alk Calc into one spreadsheet. Extracting from Bru'n Water should be simple; and I have the study used to create the math (from NCSU) that UNH later used to create their Alk Calc (in conjunction with NCSU). I am an acquaintance of a couple of professors at NCSU, so I can shoot them an e-mail if I need help with the math.
Alright man by next year (January) I will finally have a tds meter and order everything I need. I will figure this out eventually. I know it will be worth it.
but should I stick with perlite or go with sunshine mix? With sunshine mix, do you water with plain water every other time? obviously I would use ro water and add all the different acids and sillica, but not add any nutrients? as long as the ppm and ph is good?
I know these questions have to be getting old man but when you look around and read, everyone has a different answer. I just have started to trust yours more than others.
I'll answer you more completely soon, but for now, the potassium silicate isn't part of my methodology for pH buffering. The potassium silicate is used for plant nutrient formulations (or at least it should be), so I had to add it at the beginning because of the pH spike from potassium silicate. I had to account for the pH spike when doing the math with Bru'n Water (and hence with UNH Alk Calc).
I do not understand what you are asking here. Do you mean that you wish to use fertilizer(s) other than my nutrient formulations? If so, then yup, you would add the base (K2CO3) and acids, but you wouldn't have to add potassium silicate unless you wished to do so. If you do wish to use potassium silicate I suggest AgSil 16H from Custom Hydro Nutrients; you would add it after adding K2CO3, but before using Bru'n Water and before adding citric acid.obviously I would use ro water and add all the different acids and silica, but not add any nutrients? as long as the ppm and ph is good?
P.S. we give our plants silicon, not silica. It seems like a minor difference in semantics, but it's really two very different forms and plants cannot use silica as a nutrient.
I'll answer you more completely soon, but for now, the potassium silicate isn't part of my methodology for pH buffering. The potassium silicate is used for plant nutrient formulations (or at least it should be), so I had to add it at the beginning because of the pH spike from potassium silicate. I had to account for the pH spike when doing the math with Bru'n Water (and hence with UNH Alk Calc).
I do not understand what you are asking here. Do you mean that you wish to use fertilizer(s) other than my nutrient formulations? If so, then yup, you would add the base (K2CO3) and acids, but you wouldn't have to add potassium silate unless you wished to do so. If you do wish to use potasasium sialte I suggest AgSil 16H from Custom Hydro Nutrients; you would add it after adding K2CO3, but before using Bru'n Water and before adding citric acid.
I knew potassium silicate wasn't used for ph buffer. We are talking about pro-tekt right? sorry I didn't realize silica and silicate wasn't the same
I was just talking about giving plain water in sunshine mix.. Would I still add protekt and citric acid.
I know your method is much more complex than that and I will get there eventually. I want to learn the way you are doing it.
For now
This is how I have been mixing, I know I can do better but what do you think about this until i get a combo meter:
50/50 ro/tap water
ascorbic acid
2.5ml protekt
2.5ml of lemon juice from the produce section of a grocery store
4.5 ml of ph down
8ml of gh micro
14ml of gh bloom
that makes me ph between 5.5 and 6
I am just trying to find a decent method until i can afford better stuff to work with like a tds meter.
Hey all,
I thought it would be useful to post a few blog entries made by Daniel, author of Hydrobuddy. It was only after I finished my methodology that I found Daniel has written quite a bit about this topic, and even made some great mathematical simulations of pH flux under various pH buffers and concentrations of a re-circulating nutrient solution, i.e., citric acid, potassium carbonate (K2CO3) and phosphate (P).
I was originally going to use potassium bicarbonate (KHCO3) but Daniel wrote in a blog entry that using citric acid and KHCO3 together cause a lot of CO2 to form. Which of course will lower the pH due to formation of "carbonic acid" (i.e., CO2 + water). So, I decided to use potassium carbonate instead.
Daniel even uses a system similar to mine, whereby he tries to crate pH buffering using potassium carbonate and citric acid. However, his method is very crude and doesn't give datum on final alkalinity after adding ~0.01 g/L citric acid. I.e., he first adds 0.01 g/L citric acid for its pH buffering (which drops pH), then adds potassium carbonate for its pH buffering (which raises pH). He adds enough potassium carbonate to increase pH to 5.8, IIRC. However, he very well could be removing much of the alkalinity afforded by the potassium carbonate by staring with such a low pH.
The ideal range for alkalinity as ppm CaCO3 is 40-60 ppm, with 20-80 ppm being the outside range. The best way to quantify alkalinity, IMO, is to use HCO3 or mEq/L. But Bru'n Water only uses CaCO3 as alkalinity, so then must we. A few quick conversions between units of alkalinity:
A few notes:
Here are the very relevant blog entries by Daniel, I suggest people who are interested in my methodology read them:
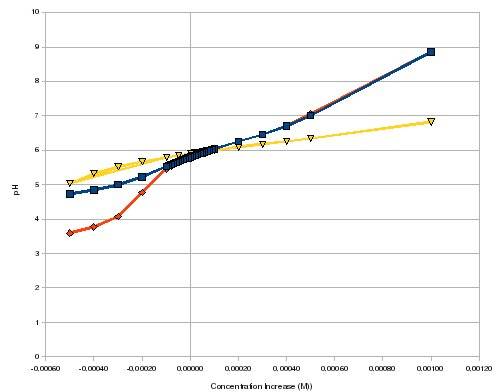
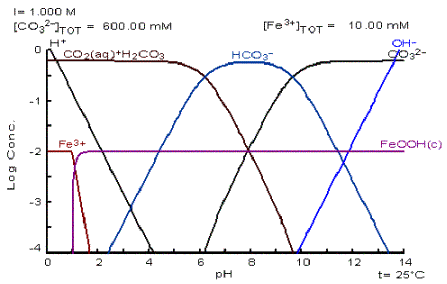
I thought it would be useful to post a few blog entries made by Daniel, author of Hydrobuddy. It was only after I finished my methodology that I found Daniel has written quite a bit about this topic, and even made some great mathematical simulations of pH flux under various pH buffers and concentrations of a re-circulating nutrient solution, i.e., citric acid, potassium carbonate (K2CO3) and phosphate (P).
I was originally going to use potassium bicarbonate (KHCO3) but Daniel wrote in a blog entry that using citric acid and KHCO3 together cause a lot of CO2 to form. Which of course will lower the pH due to formation of "carbonic acid" (i.e., CO2 + water). So, I decided to use potassium carbonate instead.
Daniel even uses a system similar to mine, whereby he tries to crate pH buffering using potassium carbonate and citric acid. However, his method is very crude and doesn't give datum on final alkalinity after adding ~0.01 g/L citric acid. I.e., he first adds 0.01 g/L citric acid for its pH buffering (which drops pH), then adds potassium carbonate for its pH buffering (which raises pH). He adds enough potassium carbonate to increase pH to 5.8, IIRC. However, he very well could be removing much of the alkalinity afforded by the potassium carbonate by staring with such a low pH.
The ideal range for alkalinity as ppm CaCO3 is 40-60 ppm, with 20-80 ppm being the outside range. The best way to quantify alkalinity, IMO, is to use HCO3 or mEq/L. But Bru'n Water only uses CaCO3 as alkalinity, so then must we. A few quick conversions between units of alkalinity:
- 1 mEq/L = 50 ppm CaCO3
- 1 mEq/L = 61 ppm HCO3
- 1 ppm CaCO3 = 1.22 ppm HCO3
- 1 ppm CaCO3 = 0.02 mEq/L
- 1 ppm HCO3 = 0.82 ppm CaCO3
- 1 ppm HCO3 = 0.016 mEq/L
- 1 dKH = 0.357 mEq/L
- 1 dKH = 17.86 ppm CaCO3
- The abbreviation "KH" is not valid for alkalinity, "KH" stands for "carbonate hardness".
A few notes:
- water with pH < 4.5 has zero alkalinity
- RO water has pH range of just below ~4.5 to just below ~7.0
- Average RO water has alkalinity of < 10 ppm CaCo3, which converts to < 12.2 ppm HCO3 and < 0.2 mEq/L
Here are the very relevant blog entries by Daniel, I suggest people who are interested in my methodology read them:
Keeping the pH of your hydroponic nutrient solution stable
February 3rd, 2009
http://scienceinhydroponics.com/200...your-hydroponic-nutrient-solution-stable.html
February 3rd, 2009
http://scienceinhydroponics.com/200...your-hydroponic-nutrient-solution-stable.html
- Please note, the "soilless solution" (thin layer of water around media particles) is the same in function as a re-circulating nutrient solution for water culture. Thus we should think of the soilless solution as if it's the reservoir in terms of pH buffering a reservoir of re-circulating nutrient solution.
My pH Balancing System for Hydroponic Growing
February 13th, 2009
http://scienceinhydroponics.com/2009/02/my-ph-balancing-system-for-hydroponic-growing.html
February 13th, 2009
http://scienceinhydroponics.com/2009/02/my-ph-balancing-system-for-hydroponic-growing.html
- Please see note above ...
Hey there Spurr, just a few questions , i see your new formulation incorporates quite a bit more Si (80-100) ppm than the previous 28.85ppm in your previous formulation.
Can you please elaborate a bit more on why you chose these specific amounts ( why not less or more ) of each element especially P, since i see it is now up from previous 52ppm to 87ppm . [ please also explain the reasoning behind the higher N (NH4+) to NO3 ration ect,]
I understand you have separate veg and flower formulas now but i thought you were researching that less P is necessary (-+50ppm). So yeah please explain more about this please ! really interested
Edit: whoops , i see in your first post you discussed my questions , patiently waiting, thanks ;p
Can you please elaborate a bit more on why you chose these specific amounts ( why not less or more ) of each element especially P, since i see it is now up from previous 52ppm to 87ppm . [ please also explain the reasoning behind the higher N (NH4+) to NO3 ration ect,]
I understand you have separate veg and flower formulas now but i thought you were researching that less P is necessary (-+50ppm). So yeah please explain more about this please ! really interested
Edit: whoops , i see in your first post you discussed my questions , patiently waiting, thanks ;p
I be taggin another Spurr thread then, G'Luck man!
I knew potassium silicate wasn't used for ph buffer. We are talking about pro-tekt right? sorry I didn't realize silica and silicate wasn't the same
For my formations I am using AgSil 16H from Custom Hydro Nutrients ("CHN" from my screen shots on page one), a really good guy who posts here and elsewhere owns that business. I really like supporting 'local folks' and his prices are very competitive. I choose to use AgSil 16H because it's not liquid and it has the lowest K:Si ratio. And it's much less expensive to use than Pro-TeKt.
... should I stick with perlite or go with sunshine mix? With sunshine mix, do you water with plain water every other time? obviously I would use ro water and add all the different acids and sillica, but not add any nutrients? as long as the ppm and ph is good?
I was just talking about giving plain water in sunshine mix.. Would I still add protekt and citric acid.
I am still a little confused as to your goal(s) and question(s), but I am very stoned right now
If you use Sunshine mix, it already has dolomitic and calcidic limes, which will add alkalinity to the soilless solution as well as increase its pH.
When I use soilless peat based media (with D and C lime*) I water will full strength (i.e., "starter solution", then the next time I water I use reduce strength of starter solution by ~50%, then the next time I water I use full strength. However, that is not a hard and fast rule.
For the automated and aerated drip irrigation system I'm designing, using tensiometer to automate opening of electric solenoid water value, I plan to run the reservoir the same way as above. That is, when I refill the reservoir I will use a dilute starter solution, probably ~40% because with automated drip irrigation by water tension, the media is watered much more often than using hand watering.
* note: gypsum don't affect pH (unless it's sodic media), although many people think it does.
Two very import considerations with watering soilless media and the elements in soilless solution (to prevent salt buildup and ion antagonisms) are: (1) percent run-off (it should be ~15% by volume to total water volume added to media); and (2) total volume of water (if you double the volume you will nearly double the ppm concentrations, we should only use as much volume as is needed for ~15% runoff, and we should use "pulse" watering method).
You may want to check out the report by Dr. Bruce Bugbee that I linked on page 1 or 2. The topics of refill solution and how quickly plants take up N vs Ca vs K vs P, etc., are important considerations.
How you fertigate depends strongly upon how often you 'water' the media ...
This is how I have been mixing, I know I can do better but what do you think about this until i get a combo meter:
50/50 ro/tap water
ascorbic acid
2.5ml protekt
2.5ml of lemon juice from the produce section of a grocery store
4.5 ml of ph down
8ml of gh micro
14ml of gh bloom
that makes me ph between 5.5 and 6
I am just trying to find a decent method until i can afford better stuff to work with like a tds meter.
That looks fine, but I would use citric acid instead of lime juice and add some Ca, Mg and S. The Mg and S are easy and cheap: Epsom salt. The Ca can be a bit more pricey and often you have to also add N via CaNO3 source of Ca. Many people use CaMag+ (from Botaincare) but it uses Fe-EDTA which is the worst choice of synthetic chelating agents. I won't use EDTA if I can help it and neither should anyone else. The great thing about DPTA, besides it's very stable over a wide pH range and doesn't degrade quickly, is it's won't bond to Ca. That is a major issue with EDTA, esp. if all metals are chelated with EDTA, that is, EDTA dropping Zn or Fe (for example) and bonding to Ca. Not only that, but EDTA is not good for plants, it's phytotoxic in sufficient (low) quantity.
Last edited:
One consideration I haven't yet noted, is the increase in alkalinity from silicon, phosphate, nitrate, borate, etc. E.x., high water pH silicon can add quite a bit of alkalinity: at pH 9.7, 100 mEq of Si adds 58 ppm CaCO3. The alkalinity from Si is the biggest unaccounted for source of alkalinity in my methodology and formulations. With the alkalinity from P a close second in full-flowering stage when P is ~87 ppm.
The math [1,2] to calculate the alkalinity from Si is too much for most growers to want to carry out. So that is why I didn't include in my methodology. That means the final alkalinity will be higher than 21.7 CaCO3. That is also why I suggest growers test the solution pH and alkalinity after adding K2CO3 and AgSil 16H (or other potassium silicate). Likewise in the case of phosphate (P).
All of the above listed elements do not affect alkalinity nearly as strongly as potassium carbonate, and the acids in my formulation. Silicon is an exception.
[1] http://books.google.com/books?id=Rj...wAw#v=onepage&q=silicate + alkalinity&f=false
[2] http://cdiac.ornl.gov/oceans/ndp_065/3d.html
The math [1,2] to calculate the alkalinity from Si is too much for most growers to want to carry out. So that is why I didn't include in my methodology. That means the final alkalinity will be higher than 21.7 CaCO3. That is also why I suggest growers test the solution pH and alkalinity after adding K2CO3 and AgSil 16H (or other potassium silicate). Likewise in the case of phosphate (P).
All of the above listed elements do not affect alkalinity nearly as strongly as potassium carbonate, and the acids in my formulation. Silicon is an exception.
[1] http://books.google.com/books?id=Rj...wAw#v=onepage&q=silicate + alkalinity&f=false
[2] http://cdiac.ornl.gov/oceans/ndp_065/3d.html
Last edited:
D
DonkDBZ
How do you think your new PH adjusted water would work with your 5/5/5/5 recipe
no need for pro tek now of course.
Also when using the preph water with stuff like pond enyzes, sweet, flora nector and so on. any guesstamated effects?
oh yeah why DAP vs MAP? dap is more solubable but cannot find online haifa map is easy to get at landscape supply
nevermind 11-62 vs 18-46 hard to get 25%nh4 and keep p low
no need for pro tek now of course.
Also when using the preph water with stuff like pond enyzes, sweet, flora nector and so on. any guesstamated effects?
oh yeah why DAP vs MAP? dap is more solubable but cannot find online haifa map is easy to get at landscape supply
nevermind 11-62 vs 18-46 hard to get 25%nh4 and keep p low
How do you think your new PH adjusted water would work with your 5/5/5/5 recipe no need for pro tek now of course.
I think it will work very well, however, the K, S and P ppm levels will be affected (which isn't a big deal). FWIW, my current GH mix is 5 mL grow/5 mL micro/7 mL bloom/5 mL CalMag+/2.5 mL Pro-TeKt/0.5 gram Epsom salt/0.015 gram boric acid (boric and fulvic acids are optional). If you use AgSil 16H your K level will increase, but that's fine.
- Here's my current formulation (as compared to he PH/Lucas formulation) for off-the-self bottles of fertilizers which is used from veg to harvest. Using General Hydropinc Flora-Series, Botnaicare CalMag+, Pro-TeKt, Espom salt and the buric acid and fulvic acid are optional:
Also when using the preph water with stuff like pond enyzes, sweet, flora nector and so on. any guesstamated effects?
On what? The plant or the water or something else, like ions?
DAP is higher purity and has less contamination than MAP, there are industry standards for DAP and not so for MAP. That is why I choose DAP and not MAP.oh yeah why DAP vs MAP? dap is more solubable but cannot find online haifa map is easy to get at landscape supply
nevermind 11-62 vs 18-46 hard to get 25%nh4 and keep p low
You can find DAP on Ebay for the same price as MAP. The owner of Custom Hydro Nutrients (CHN) told me he is planning to stock DAP (he now only sells MAP), as well as every other substance in my formulations. But it will be some months. For now, most of the substances for my formulations come from CHN and JR Peters.
Last edited:
Hey there Spurr, just a few questions , i see your new formulation incorporates quite a bit more Si (80-100) ppm than the previous 28.85ppm in your previous formulation.
Yup. I am going to use 80 ppm Si in veg, which is 2.848 milliMolar/L Si and 273.6 ppm "orthosilicic acid" (H4SiO4; the plant usable form of silicon). I am going to use 90 ppm Si in early-flowering (and nearly that in full-flowering), which is 3.204 mmol/L Si and 307.8 ppm orthosilicic acid, aka "monosilicic acid" aka "silicic acid". A commonly used concentration of Si in nutrient solutions is 100 ppm, some people claim it's 100 ppm SiO2, but that's incorrect. It's a claim made from an aged report on silicon and plants.
For hydroponic research it has been found upwards of 5 to 10 mM Si can be beneficial, esp for PM and heat issues. And those values convert to 140.43 ppm to 280.86 ppm Si. Plants commonly have higher Si content (by tissue assay) than Ca, Mg, S, P, even total N in some cases. Plants can use lots of Si without problems.
A few conversions I made to help:
- 1 mmol/L Si = 28.0855 ppm Si
- 1 ppm Si = 0.0356 mmol/L Si
- 1 mmol/L H4SiO4 = 96.115 ppm H4SiO4
- 1 ppm H4SiO4 = 0.01 mmol/L H4SiO4
- 1 ppm Si = 3.42 ppm H4SiO4
- 1 ppm H4SiO4 = 0.292207 ppm Si
- and two useful equations:
- (mmol/L)molar mass = ppm
- (mg/L)/molar mass = mmol/L
One reason I used ~1 mmol/L Si (i.e., ~1 mM Si) with Pro-TeKt is the price, and I'm not sure how accurate the data is for % K20 and % SiO2 on Pro-TeKt's label. Currently my formulations use between 2.848 mmol/L Si to 3.204 mmol/L Si.
I wanted to use less than 100 ppm of Si, but to keep S (sulfate) below 110 ppm. That meant I had to increase the AgSil 16H (in early-flowering) to (at least) 90 ppm Si.
I increased Si during vegetative phase, from my previous ppm of ~30, because further testing on cannabis shows plants respond well to > 30 ppm. Many cannabis growers use 100 ppm SiO2, which converts to 46.7435 ppm Si. I also increased Si because it seems more people are having issues with PM, and increasing Si will help hinder PM.
I increased Si in early-flowering and full-flowering because of the increased cell growth/elongation vs vegetative stage. As well as because trichome of the Genus Cannabis are highly "silicaifed", that is, trichomes are compromised of much silicon (or would it be silica?), possibly as SiO2 (silicon dioxide), the common form of silicon once H4SiO4 is 'absorbed' and converted by the plant. Plants 'take in' orthosilicic acid (H4SiO4) and then convert it to SiO2, and maybe other forms of which I'm unaware. I also increased Si do help with PM in flowering stages.
- Here are some good screenshots from a couple of studies and rep reports:
----------------------------------------------
----------------------------------------------
----------------------------------------------
----------------------------------------------
We give our plants silicon, not silica. I would not wish to use any product that's potassium silicate and has "silica" in the label, ex., "Silica Blast". If the manufacturer doesn't understand that key difference I don't have much faith in the quality their product.
Can you please elaborate a bit more on why you chose these specific amounts ( why not less or more ) of each element especially P, since i see it is now up from previous 52ppm to 87ppm.
I understand you have separate veg and flower formulas now but i thought you were researching that less P is necessary (-+50ppm). So yeah please explain more about this please ! really interested
In vegetative and "early-flowering" stages I am using 30 ppm P. In "full-flowering" stage I am using 87 ppm P. I increased the P from 30 ppm (sufficient range, albeit a bit high) to 87 ppm after pre-flowering stretch and after initial flower-set, ie., what I term "early-flowering" stage. Once plants are past initial flower-set, the stage I term "full-flowering" stage, I increase P because doing so may increase THC.
I have read one good study, and a second okay study, on effects from various application rates of N, P, K, etc., on THC. In one good study, the researchers found increased P (and reduced K) was strongly correlated to increased THC. The authors hypothesized increased P may affect enzyme pathway thereby increasing THC-A, IIRC. However, they did not quantify glandular trichome density (trichs per mm^2), they only carried out quantification assay on extract. I personally feel increased P may increase trichome density, due to the reports by other cannabis growers; if so, the THC quantity would also increase.
In the future I plan to carry out very well designed and controlled studies to see if increasing P does increase THC-A and trichome density. For now I am covering my bases by increasing P.
The biggest reason to keep P at sufficient level of ~20-40 ppm is to reduce internodal elongation, and to increase root growth (i.e., reduce the "root:shoot ratio"). By the time full-flowering stage arrives the roots are pretty much done growing (elongating), as are shoots.
I plan to test 20 ppm P with use of AM (arbuscular mycorrhizae) fungi. I have written very much on this topic, and I'm the one who blew apart the myth about high efficacy of AM fungi in cannabis growth with P greater than ~30 ppm. If we plan to use AM fungi we need to keep P below 25 ppm, ideally below 20 ppm.
[ please also explain the reasoning behind the higher N (NH4+) to NO3 ration ... ]
As we know, ion ratios are about antagonisms and potentiations, so they are quite important. As are the sources of elements, which is why I will not use MAP (mono-ammonium phosaphte) if I can help it, and will instead use DAP (di-ammonium phosphate). DAP is higher purity and has less contamination than MAP, there are industry standards for DAP and not so for MAP. That is why I choose DAP and not MAP.
I choose the ratio of 4, for NO3:NO4, because of the buffering effect on pH (from lower ratio) and impact on plant usage of increased CO2 from ammonium (NH4). I kept the ratio of 4 for all stages of growth. Ideally, a ratio of 3 is better in terms of pH and CO2 (re ppm of NH4). However, plants in warm conditions and bright light, esp. flowering and fruiting plants where the "photosyntate" stays close to the source tissue (i.e., leafs and flowers) the plant is less able to move carbohydrates (sugar) to roots to convert NH4 into amino acids. In such a case a ratio above 3 is ideal to prevent phytotoxicity and poor growth.
In terms of effect from NO3:NH4 ratio on pH in rhizosphere and soilless solution, or reservoir (for water culture), we must consider root exudates. As roots 'take in' the anion nitrate (NO3), they release (exude, re exudate) bicarbonates, etc., which increases pH (as well as alkalinity), however, when roots 'take in' the cations ammonicial nitrogen (ammonia (NH3) and ammonium (NH4), they release H+ protons which reduce pH and alkalinity. Thus, by reducing the NO3:NH4 ratio (when all ammonicical N is NH4) close to 1, we in effect reduce pH swings by better balancing acidic and basic exudates from roots.
However, one must consider the process of "nitrification", whereby NH4 is converted into nitrite (NO2) and NO2 is then converted into NO3 (nitrate). This process happens quite fast and has high efficacy when there are copious microbes able to carryout the conversion, such as "nitrifying bacteria" species, which are highly ubiquitous. That means unless we use something like chlorine dioxide at 0.25-0.5 ppm or hydrogen peroxide, much NH4 will be converted into NO3, thereby increasing the NO3:NH4 ratio.
There is also the topic of antagonisms and potentiations of NO3, by NH4. That is, at first (couple of house) NH4 potentiates uptake of NO3, however, after some times (hours/days) NH4 antagonizes uptake of NO3. I used a NO3:NH4 ratio which should reduce the effects of NH4 on NO3, vs ideal ratio of 3 (in terms of pH).
In terms of the long term effects of increased CO2 the higher the NH4 ppm the better, within reason. Please see this thread I wrote about CO2 for much more information on this topic: The facts about CO2 ppm: don't use 1,500!
- Here is a screenshot from a good report showing the process of nitrification (left hand side) and the effect of urea and various ionic species of nitrogen on root exudates and thus upon pH and alkalinity:
----------------------------------------------
[ please also explain the reasoning behind ... ration etc. ]
I choose a K:Mg ratio of 3 because the ppm of K has a stronger effect (antagonization) of Mg than Ca. I kept the ratio of 3 for K:Mg in all stages of growth. I would have kept a ratio of 1.5 for K:Ca, however, that would have increased NO3 too far and messed up my NO3:NH4 ratio.
I try to use at least a ratio of 2.5 for K:Ca, and high ppm Ca because plants 'take up' Ca slower than almost every other element, and Ca-P precipitation can/will form in rhizosphere (AFAIK) thus reducing available Ca.
I boosted K to 230 ppm in early-flowering (and boosted Mg to keep the ratio of 3), which may or may not help yield. Many growers believe boosting K can increase yield, myself included. I reduced K back to ~200 ppm for full-flowering just in case increased K can reduce THC. Using the fertilizer compounds I choose, I can boost K but not increase P at all
I choose a Ca:Mg ratio of equal to or less than 2 because that ratio has worked very well for me and many other growers.
I choose high S (sulfate) because it's my "degree of freedom" element in HydroBuddy, thereby allowing me to create a more accurate formulation. Also, sulfate is commonly used up to ~120 ppm in academic and commercial hydroponics. My goal was to keep S below 110 ppm. Also, because S affects smell I increased S to help increase terpene action to increase smell (I'm not positive of the chemical steps or the science). I decreased S during full-flowering to affect the P:S ratio, to reduce possible antagonism of P by S.
Okay, I think that about covers the most important topics. If I forgot something please feel free to let me know.
NOTE:
As plants near time to harvest I start decreasing EC, as reported by NSCU FloraCulture "PourThru" methodology (when using soilless - the PourThru method isn't need for water culture). Using any other method, ex., haphazardly testing run-off as most growers do, provides very inaccurate EC results. That means nearly every soil and soilless cannabis grower doesn't now the real EC of the rhizosphere and soilless solution unless they use PourThru (or other proven methods*). However, use of "suction lysimeters" allow for even greater accuracy of EC and pH from soilless soition ...
* Other methods to accurately test EC and pH of soilless solution are a no go for living plants, ex., "SME", "1:2", "1:5", etc. Also, due to differences in each methodology one must use conversion factors to compare EC from NCSU PourThru to SEM to 1:2, etc.
Last edited:


