I get mine from ebay..
http://www.ebay.com/itm/50-Micron-S...t=LH_DefaultDomain_0&var=&hash=item76bf9d0123
http://www.ebay.com/itm/50-Micron-S...t=LH_DefaultDomain_0&var=&hash=item76bf9d0123
Joe I believe there just getting everything below freezing before they start there extraction. There not exactly freezing any butane.
well technically colder temps should make for a better extraction, which is a reason alot of ppl freeze the plant material before they use it
dont know how cold you can get the butane with a reg freezer, would be nice to have temp readings of a bottle in the freezer and one right off the shelf, but i dont knowhowone would go about measuring the temps as room temps would surely affect it
i would just hope that with the pressureized can in the freezer wont explode

If I understand you correctly,dont know how cold you can get the butane with a reg freezer, would be nice to have temp readings of a bottle in the freezer and one right off the shelf, but i dont knowhowone would go about measuring the temps as room temps would surely affect it
I do not think the experiments a waste of time. Even I will not waste words on explaining why I think so.all these experiments, although fun to do, are just a waste of time.
I don't really see how freezing the cans of butane will help the extraction.
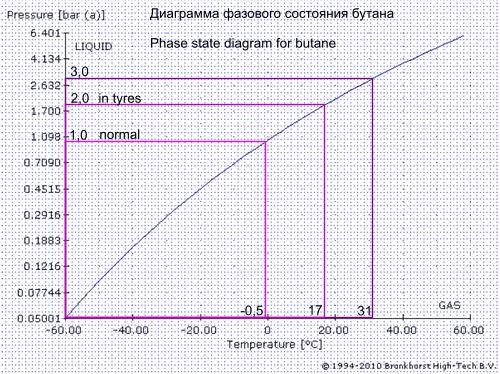
At the exit from the can gas pressure decreases and becomes insufficient to maintain the gas in the liquid state, gas is forced to evaporate.room temperature butane and weed is fine,and butane is not room temperature anyway, its much colder already, freezing, to be a liquid.

The denser packing and (or) filter, the higher pressure develops inside the tube and the higher temperature of the liquid butane.What about the temp of the solvent? I know for a fact that heated everclear is a much stronger solvent than cold everclear and cold everclear is more selective than hot everclear. Now I don't know this for sure but I think the same would be true with butane.
huge skills!If I understand you correctly,

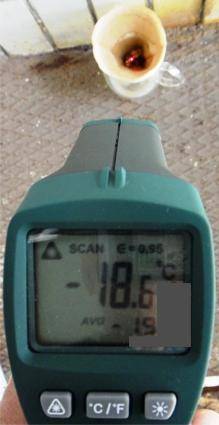
I do not think the experiments a waste of time. Even I will not waste words on explaining why I think so.
But in this case it is not about the experiments, but the knowledge and application of physical laws discovered by other experimenters long ago before us.