I doubt the water being magnetized has anything to, or what many people consider to be water structures in groups. Water molecules are normally carried one molecule or mol at a time by different highly controlled enzymes. The way that they are structured as a group would not play a role since most molecules in a cell are highly controlled by enzymes specific for one molecule at a time. The limiting reagent in most photosynthetic complexes is the free electron. If anything magnetic field actually constrains water molecules by making them bind to each other more tightly. that might be why your yields are lower for the magnetic group. See this link for a study done of magetic field on water.
Reading this study, it actually seems that Fever has a point.
"It has been reported that the
dissolution rate into water of some materials, e.g., oxygen
and copper sulfate, is significantly accelerated by the presence
of a magnetic field."
As you will know, plants do not actively take in oxygen (rather they do it via diffusion or production during photosynthesis) so I can see how this would be a benefit.
"The effect of the magnetic field in enhancing
the hydrogen bonding was confirmed by Inaba et al."
"Specifically, as the strength of the magnetic field
increases from 1 to 10 T, the number of hydrogen bonds increases
by approximately 0.34%. This slight increase in the
number of hydrogen bonds indicates that the magnetic field
enhances the water networking ability."
Again this supports Fever's idea, increased hydrogen bonding is going to make the water more able to form solutions with hydrophillic molecules/ hold more in solution. This *could* mean greater input of nutrients.
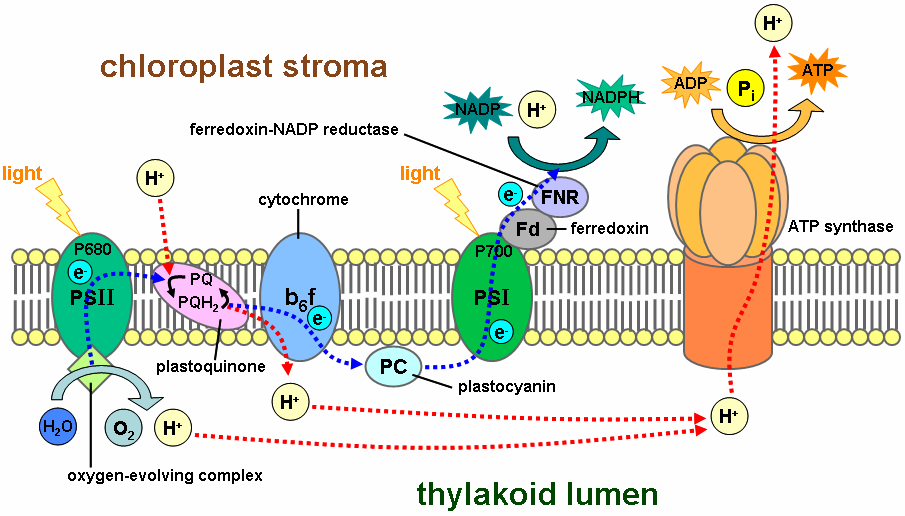
The negative electrons going directly to photosystem I and II which start the chain enzyme reaction to split H20 to provide the H+ molecule which then increases the voltage gradient. So I believe by adding electrons through current you are supplementing the electrons which are usually acquired by photosynthesis, which could have some really interesting effects on the expression of certain genes.
Woah woah step back a bit. First off, Photosystem I simply carries the electron to ferredoxin to complete the reduction of NADP+ to NADPH. Photosystem II splits 2 water molecules into an O2 molecule, 4 free electrons (which pass down the photosystem until they reach photosystem I to reduce NADP+) and 4 H+ ions (protons) which are then used to drive ATP synthase via the [chemical] voltage gradient. A magnet wouldn't 'add' electrons so much as potentially alter their energy level or movements, which could affect the whole system. You're describing what would happen if Trich was feeding an electrical current into his plants. This wouldn't [I don't think anyway, hardly an expert on plant genetics] affect genetic expression directly except perhaps by some kind of absent signal or by affecting other proteins as I previously described.
Trich: Interesting results mate! I did a quick stats test (t-test), you have a significance of p=0.17. Typically p<0.05 is a significant result but p<0.25 is pretty decent given the sample sizes! This is such an interesting project