nothing's finer. 


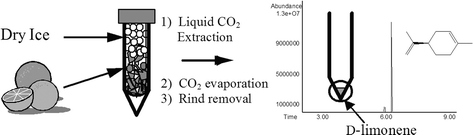
A unique liquid CO2 extraction laboratory developed for a greener organic teaching lab curriculum provides an effective, inexpensive, and convenient procedure for teaching natural products extraction concepts and techniques using modern green extraction technology. The procedure is appropriate for the teaching lab, does not require any special equipment, and allows the students to see the phase change and extraction as they occur. Students learn extraction and spectroscopic analysis skills, are exposed to a dramatic visual example of phase change, and are introduced to commercially successful green chemical processing with CO2.

The graph you've posted seems to agree that C02 reaches critical state at 31.1C/88F and 72.9 atmospheres, or about 1086 PSI.
By agreement, pressure cookers vent excess pressure at 15 psi (about ~1 atmosphere), so assuming it has been replaced by a 1100 psi back pressure regulator, what is the burst pressure of the pressure cooker that you are using?
Have you also checked your material selection at the cryogenic temperatures that you are operating at when you release the pressure? Some materials, such as carbon steel, turn into fragmentation grenades at cryogenic temperatures.
......1086 psi you say?1 atm = 14.6959488
Liquid CO2 forms at 5.1 atm or 74.94933888 psi.
(A little data for those looking to try this, use it however you will)

LOL. Plans for a grand CO2 extractor based on a loose-capped centrifuge tube that leaks gas so hard that it spins at mad RPM in its water bath. Now, let's talk split ring non-stirred reactors.

They ain't cheap, so people can stop asking the great "heady blunts" for an inexpensive DIY.

......1086 psi you say?
I guess that means I will need to use a pressure paint pot to hold the extraction. I thought Pressure cookers could hold up to 80 psi but I found they only hold about 15-20psi then fail to work, so no boom, just failure to do anything at all.
And there's a little "science" from your ethanol extraction Messiah. Gray Wolf, why would people be keeping their CO2 tanks at 216.592 Kelvin? After all, it's not until that low temperature that saturated carbon dioxide's pressure decreases to 0.51796 MPa (Span and Wagner 1996). At room temperature, everyone knows the pressure is more akin to 5.1 MPa, the figure you have decided to change to ATM / Bar for no good reason. There's a lesson for you, kids: using the royal "we" doesn't make someone know science.1 atm = 14.6959488
Liquid CO2 forms at 5.1 atm or 74.94933888 psi.
(A little data for those looking to try this, use it however you will)
And there's a little "science" from your ethanol extraction Messiah. Gray Wolf, why would people be keeping their CO2 tanks at 216.592 Kelvin? After all, it's not until that low temperature that saturated carbon dioxide's pressure decreases to 0.51796 MPa (Span and Wagner 1996). At room temperature, everyone knows the pressure is more akin to 5.1 MPa, the figure you have decided to change to ATM / Bar for no good reason. There's a lesson for you, kids: using the royal "we" doesn't make someone know science.
At 298 Kelvin, saturated CO2 will possess a pressure of 6.4121 MPa. The fact that I actually have to explain this suggests, as I initially stated, that I only care about talking to foaf.
tldr; Gray Wolf forgot to multiply by 10. Liquid CO2 is fuck-high pressure too.
so can anyone tell us ignorant non scientists the difference between co2 extraction and super critical co2 extraction? in plain old english. is the super critical referring to doing it under pressure?