easystephen
Member
I recently bought the Across International 50L rotovap but the chiller that they bundle with it seemed underpowered. I wanted to share some calculations I did to figure out what power chiller I would actually need to get the 20L/hr throughput rate they spec. You can modify for whatever unit you might be considering.
Enthalpy change of condensation -38.56 kJ/mole
Density 0.79 g/ml
Molar mass 46.10 g/mole
Liters per mole 0.06 L/mole
Enthalpy change per liter -659.95 kJ/L
Condensation rate 20.00 L/hr
Time per liter 180.00 s/L
Cooling power needed -3.67 kW
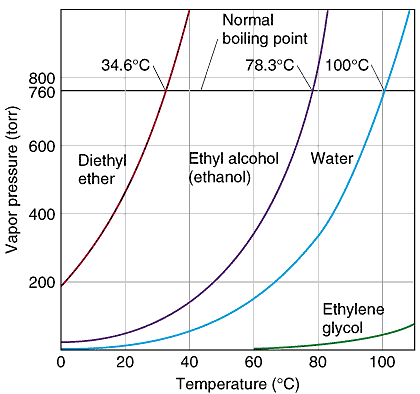
So, in order to condense 20L/hr I need a chiller with 3.67 kW of cooling power at a temperature of around 20C less than the boiling point of ethanol at the pressure in the rotovap. So if I want to be able to distill down to 75 Torr I need 3.67 kW at around 0C. The specs on the chiller from AI say it has 2.8 kW at 0C which clearly doesn't cut it. In fact, 3.67 kW at 0C probably won't cut it either since the fluid warms up in the condensing coils as it pulls energy from the ethanol gas so we really want 3.67 kW at something like -10C.
Enthalpy change of condensation -38.56 kJ/mole
Density 0.79 g/ml
Molar mass 46.10 g/mole
Liters per mole 0.06 L/mole
Enthalpy change per liter -659.95 kJ/L
Condensation rate 20.00 L/hr
Time per liter 180.00 s/L
Cooling power needed -3.67 kW
So, in order to condense 20L/hr I need a chiller with 3.67 kW of cooling power at a temperature of around 20C less than the boiling point of ethanol at the pressure in the rotovap. So if I want to be able to distill down to 75 Torr I need 3.67 kW at around 0C. The specs on the chiller from AI say it has 2.8 kW at 0C which clearly doesn't cut it. In fact, 3.67 kW at 0C probably won't cut it either since the fluid warms up in the condensing coils as it pulls energy from the ethanol gas so we really want 3.67 kW at something like -10C.