----------------------------
you have acess to cbd in your country,? even in a pure form, GREAT!
but its not psychoactive....damnnnnn
what would you say when i tell you how to convert your cbd into delta-8-THC !
because of this post from wolf, lol back in 2009.....
https://www.icmag.com/ic/showthread.php?t=184612
i took a look into cannabinoid chemistry and i found a patent and i improved it,
at least i think so.
its about how you "cook" cbd into,only delta-8, or both delta-9 and 8 THC mixture,
depends on the catalyst which is used.
patent:
https://patents.google.com/patent/US20040143126A1/en
if there where any organic chemistry knowledged people around, tell me what YOU think.
i will post the original patent here,
my improved version of it (you need chemistry equipment)
one pot reaction (easy, well, not so pure)
and then do the reaction by myself.
delta-8-thc is less potent like half strength or so (the literature says 1/3 or 1/2 strenght, i dont know)
but if you get pure CBD crystalls you may not care because it will be STRONG.
the yield should be great and purity also when doin' correctly.
i got a friend working in a lab and she may be helping and do HPLC analysis!!!
this has to be done seriously ! no chick chack maybe yes maybe no....
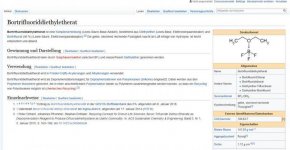
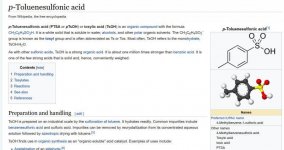
if you change catalyst you get delta-8-thc and delta-9-thc,
which is more psychoactive but the catalyst is impossible to be made by yourself,
so if you cant buy it then you cant do it. the delta-8-thc catalyst you can buy easy or DIY.
I'M NOT READY YET !
ok and now even more interesting.
wolf called it THC-Acetate (THC-O-Acetate)
well, in fact this is a cannabinoid-ester which is also easy made and should,
improve psychoactivity, a lot!
stronger or more psychedelic effects.
i think this is because it crosses the blood-brain barrier easier, when it's in acetate form.
just look for THC-O-Acetate (talkin' bout delta-9)
the first reciepe he posted is just improved when you take clean alcohol (water-free) and glacial acetic acid....
so even this should work to get the ester. this is important because of restrictment of acetic anhydride.
which make your weed much stronger and more psychoactive as far as written, in literature.
i didnt try any of these reactions, nor i took some ester's or other changed structures of cannabinoids so far, so
i cant tell you exactly.
please put this in your mind.
i never saw a delta-8-thc-ester (the acetate), or any information about it. (prooved)
it s possible to get other carbon acids (formic acid) to react and nobody knows
whats happening then, WTF
you could even add to the carbon acids other groups, but this would be really too much too discuss in here.
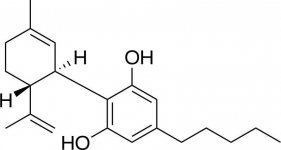
if you look into the structure of cbd you could even hang a group on the double bond... bla bla FACT:
GOUVERMENT WILL HATE ME, AND YOU TOO !
but well there were patents so... fuck'em
PS: this is not my achievment and its done way, way before i looked at something like that.
maybe wolf jumps in here i'm sure he could give prooved information about my suggestion and so,
but maybe he want to keep this information for himself
be aware when you buy acetic anhydride (its impossible to cook heroine without it!)
you may get watched by authorities.
SECURITY IS A MUST.
i add the structure of the 3 most (former) important cannabinoids.
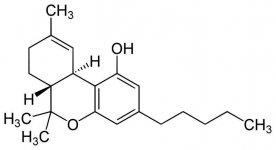
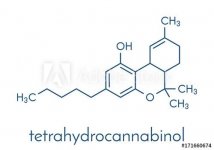
the diffrence between delta-8 and delta-9 THC is just the double bond in the ring.
i didnt do so much research but it may be possible ( if the delta 9 is more stable one) to convert even delta-8 THC into delta-9
you have acess to cbd in your country,? even in a pure form, GREAT!
but its not psychoactive....damnnnnn
what would you say when i tell you how to convert your cbd into delta-8-THC !
because of this post from wolf, lol back in 2009.....
https://www.icmag.com/ic/showthread.php?t=184612
i took a look into cannabinoid chemistry and i found a patent and i improved it,
at least i think so.
its about how you "cook" cbd into,only delta-8, or both delta-9 and 8 THC mixture,
depends on the catalyst which is used.
patent:
https://patents.google.com/patent/US20040143126A1/en
if there where any organic chemistry knowledged people around, tell me what YOU think.
i will post the original patent here,
my improved version of it (you need chemistry equipment)
one pot reaction (easy, well, not so pure)
and then do the reaction by myself.
delta-8-thc is less potent like half strength or so (the literature says 1/3 or 1/2 strenght, i dont know)
but if you get pure CBD crystalls you may not care because it will be STRONG.
the yield should be great and purity also when doin' correctly.
i got a friend working in a lab and she may be helping and do HPLC analysis!!!
this has to be done seriously ! no chick chack maybe yes maybe no....
if you change catalyst you get delta-8-thc and delta-9-thc,
which is more psychoactive but the catalyst is impossible to be made by yourself,
so if you cant buy it then you cant do it. the delta-8-thc catalyst you can buy easy or DIY.
I'M NOT READY YET !
ok and now even more interesting.
wolf called it THC-Acetate (THC-O-Acetate)
well, in fact this is a cannabinoid-ester which is also easy made and should,
improve psychoactivity, a lot!
stronger or more psychedelic effects.
i think this is because it crosses the blood-brain barrier easier, when it's in acetate form.
just look for THC-O-Acetate (talkin' bout delta-9)
the first reciepe he posted is just improved when you take clean alcohol (water-free) and glacial acetic acid....
so even this should work to get the ester. this is important because of restrictment of acetic anhydride.
which make your weed much stronger and more psychoactive as far as written, in literature.
i didnt try any of these reactions, nor i took some ester's or other changed structures of cannabinoids so far, so
i cant tell you exactly.
please put this in your mind.
i never saw a delta-8-thc-ester (the acetate), or any information about it. (prooved)
it s possible to get other carbon acids (formic acid) to react and nobody knows
whats happening then, WTF
you could even add to the carbon acids other groups, but this would be really too much too discuss in here.
if you look into the structure of cbd you could even hang a group on the double bond... bla bla FACT:
GOUVERMENT WILL HATE ME, AND YOU TOO !
but well there were patents so... fuck'em
PS: this is not my achievment and its done way, way before i looked at something like that.
maybe wolf jumps in here i'm sure he could give prooved information about my suggestion and so,
but maybe he want to keep this information for himself
be aware when you buy acetic anhydride (its impossible to cook heroine without it!)
you may get watched by authorities.
SECURITY IS A MUST.
i add the structure of the 3 most (former) important cannabinoids.
the diffrence between delta-8 and delta-9 THC is just the double bond in the ring.
i didnt do so much research but it may be possible ( if the delta 9 is more stable one) to convert even delta-8 THC into delta-9
Attachments
-
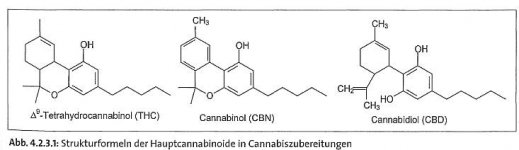 3mostimportantcannabinoids.jpg19.2 KB · Views: 34
3mostimportantcannabinoids.jpg19.2 KB · Views: 34 -
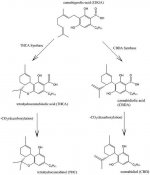 516px-Cannabidiol_and_THC_Biosynthesis.jpg23.4 KB · Views: 39
516px-Cannabidiol_and_THC_Biosynthesis.jpg23.4 KB · Views: 39 -
 best_cbd_thc9_catalyst.jpg47.3 KB · Views: 36
best_cbd_thc9_catalyst.jpg47.3 KB · Views: 36 -
 delta9thcmolekül.jpg13.1 KB · Views: 32
delta9thcmolekül.jpg13.1 KB · Views: 32 -
 delta-8-thc-mole_1.jpg72.3 KB · Views: 25
delta-8-thc-mole_1.jpg72.3 KB · Views: 25 -
 delta8_catalyst.jpg47.4 KB · Views: 33
delta8_catalyst.jpg47.4 KB · Views: 33 -
 cbd_isomere.jpg36.2 KB · Views: 30
cbd_isomere.jpg36.2 KB · Views: 30
Last edited: