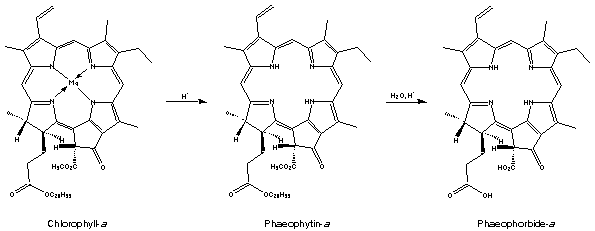
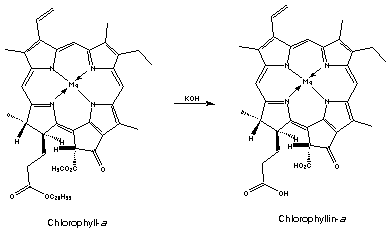
Suggested by Pharmer Joe for post extraction removal of chlorophyll :
Materials needed:
Acetonitrile
Acetone
Graphite
Flash silica
Fritted funnel
Erlymeyer flask with vacuum side arm
Vacuum pump
Procedure
Dissolve oil in acetonitrile
Add 5% (oil wt) graphite and stir 1 hour
Make slurry with silica and acetonitrile
Set up filter apparatus
Pour silica slurry onto fritted funnel
When the solvent drains just above the bed surface; pour graphite mixture and apply gentle vacuum.
Clean equipment up afterwards with Acetone.
Materials needed:
Acetonitrile
Acetone
Graphite
Flash silica
Fritted funnel
Erlymeyer flask with vacuum side arm
Vacuum pump
Procedure
Dissolve oil in acetonitrile
Add 5% (oil wt) graphite and stir 1 hour
Make slurry with silica and acetonitrile
Set up filter apparatus
Pour silica slurry onto fritted funnel
When the solvent drains just above the bed surface; pour graphite mixture and apply gentle vacuum.
Clean equipment up afterwards with Acetone.