-
Happy Birthday ICMag! Been 20 years since Gypsy Nirvana created the forum! We are celebrating with a 4/20 Giveaway and by launching a new Patreon tier called "420club". You can read more here.
-
Important notice: ICMag's T.O.U. has been updated. Please review it here. For your convenience, it is also available in the main forum menu, under 'Quick Links"!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Cannabis Seed Storage
- Thread starter acespicoli
- Start date
acespicoli
Well-known member
Method 2
Freeze Drying with Dry Ice
-

1
Wash the produce you want to freeze dry and cook any meat. Wash fruit and vegetables under cool running water before patting each item dry with a paper towel. If you’re drying poultry, beef, or fish, make sure to cook them first.[13]- Pasta noodles should be cooked as well.
- You don’t need to wash cheeses before you freeze dry them.
-

2
Slice larger items into chunks about 1–2 inches (2.5–5.1 cm) across. Use a sharp knife to slice larger fruit and vegetables into small chunks about 1 inch (2.5 cm) to 2 inches (5.1 cm) across. If you’re freeze drying cooked meat, slice it into slivers less than 1 inch (2.5 cm) thick. Try to make each piece the same size so they freeze dry at the same rate.[14]- Small fruits like blueberries, raspberries, and blackberries can be freeze dried whole.
- Slice larger pieces of produce like potatoes, apples, and pears into smaller chunks.
- If you’re freeze drying a loaf of bread, use a serrated knife to cut it into slices about 1⁄2 inch (1.3 cm) thick.
-

3
Put the chopped food chunks into freezer bags and seal the bags. Put the sliced chunks into freezer bags. Be sure to put only 1 type of food per bag rather than mixing different kinds of foods together. Then, push out all of the air from the bags with your hands or by rolling the air out (toward the opening) with a rolling pin.[15]- Pushing out the air will ensure that no ice crystals form on the food.
-

4
Choose a storage box large enough that the bags only fill it half way. A large styrofoam cooler or large plastic container with a lid will work nicely. Note that the box will have to fit inside of your freezer, so if you have a small freezer, you may only be able to freeze dry small quantities of food at a time.[16]- Pick a plastic container you don’t plan to use for other purposes because you’ll need to put holes in the lid.
-

5
Pour 1 pound (0.45 kg) of dry ice into the bottom of the box. Put on heavy duty gloves like leather or work gloves to pour dry ice over into the bottom of the box until it forms an even layer. The amount of dry ice you need to use is equal to the weight of the food. So if you’re freezing 5 pounds (2.3 kg) of food, you’ll need about 5 pounds (2.3 kg) of dry ice.[17] If it doesn’t cover the entire bottom of the box, add another 1 pound (0.45 kg) until it does.- Depending on the width and length of the box, 5 pounds (2.3 kg) of dry ice should be enough for up to 4 layers of food.
- Don’t touch the dry ice with your bare hands—it will burn your skin! If you don’t have heavy duty or leather gloves, use oven mitts or thick kitchen towels.
- Purchase dry ice cubes online or at your local grocery store or supermarket.
-

6
Sandwich the food bags between layers of dry ice. Layer the bags on top of the bottom level of dry ice and then pour in another 1 pound (0.45 kg) to 2 pounds (0.91 kg) of dry ice to completely cover the bags. Make sure not to stack two bags directly on top of each other.[18]- You may need to rearrange the pieces of dry ice so that the bags are fully covered.
- Make sure each bag lays as flat as possible and that there’s no overlapping.
-

7
Add a final layer of dry ice on top of the food bags. Depending on the size of the box and the number of bags you have, you may need to do a few alternating layers of dry ice and food bags. Each layer of food should have dry ice on top of and underneath it.[19]
-

8
Poke a few holes into the lid and attach it to the box. Use a box cutter or sharp knife to cut 3 to 4 holes into the top of the box. These holes allow gas and moisture to escape, which is necessary for the dry ice to dissipate and for the food to fully dry.[20]- Avoid poking too many holes into the lid. The idea is to allow the gas to escape at a relatively slow rate.
-

9
Place the box into the freezer for at least 24 hours. The food is done freezer drying when all of the dry ice has disappeared. This could take 24 hours or more depending on how many layers of food you’re freeze drying (and how much dry ice you’ve used to cover it). Wear gloves to remove the lid of the box and look into the container.[21]- If you don’t see any dry ice on top, shuffle the bags around with a gloved hand to check for dry ice on the bottom. If it’s all gone, the food is ready for storage.
- If you see any chunks of dry ice, reattach the lid, reinsert the box into the freezer, and wait for 3 to 6 hours before checking again.
-

10
Store freeze-dried foods in freezer bags at room temperature. Since the foods are already in freezer bags, you can just take them out and put them in your pantry or anywhere that’s at or below room temperature.[22]- The freeze-dried food will stay good for up to 25 years.
- Eat the freeze-dried chunks as is or rehydrate them by placing them in a small amount of water.

acespicoli
Well-known member
acespicoli
Well-known member
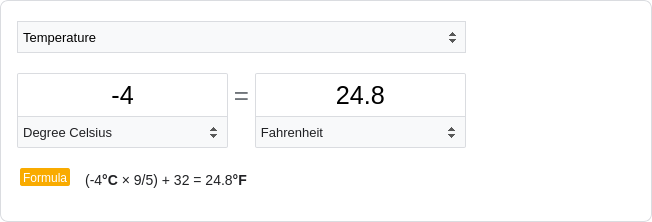
Pollen stored
under freezer conditions (-4˚C) maintained high non-abortion rates even after 96 weeks of
storage, and the associated linear model predicted that pollen stored under freezer conditions
may maintain some intact regenerative nuclei up to 261.5 weeks, approximately five years after
anther dehiscence

 doi.org
doi.org


 en.wikipedia.org
en.wikipedia.org
under freezer conditions (-4˚C) maintained high non-abortion rates even after 96 weeks of
storage, and the associated linear model predicted that pollen stored under freezer conditions
may maintain some intact regenerative nuclei up to 261.5 weeks, approximately five years after
anther dehiscence
Methods for characterizing pollen fitness in Cannabis sativa L.
Pollen grains are male gametophytes, an ephemeral haploid generation of plants, that commonly engage in competition for a limited supply of ovules. Since variation in reproductive capabilities among male gametophytes may influence the direction and pace of evolution in populations, we must be...

Fractal - Wikipedia
Last edited:
acespicoli
Well-known member
Pollen stored under room temperature conditions (22 ± 0.95˚C) quickly declined in
viability, reaching 0% germination two weeks after anther dehiscence.
PLOS ONE | https://doi.org/10.1371/journal.pone.0270799 July 7, 2022
viability, reaching 0% germination two weeks after anther dehiscence.
PLOS ONE | https://doi.org/10.1371/journal.pone.0270799 July 7, 2022
acespicoli
Well-known member
The linear regressions used to model the relationship between storage time and abortion rates
predicted that all pollen grains would degrade at
38.3 weeks under room temperature conditions (Adj. R2 = 0.8903),
and
261.5 weeks under freezer conditions (Adj. R2 = 0.8991),
suggesting that long-term storage of pollen samples for genotyping is feasible.
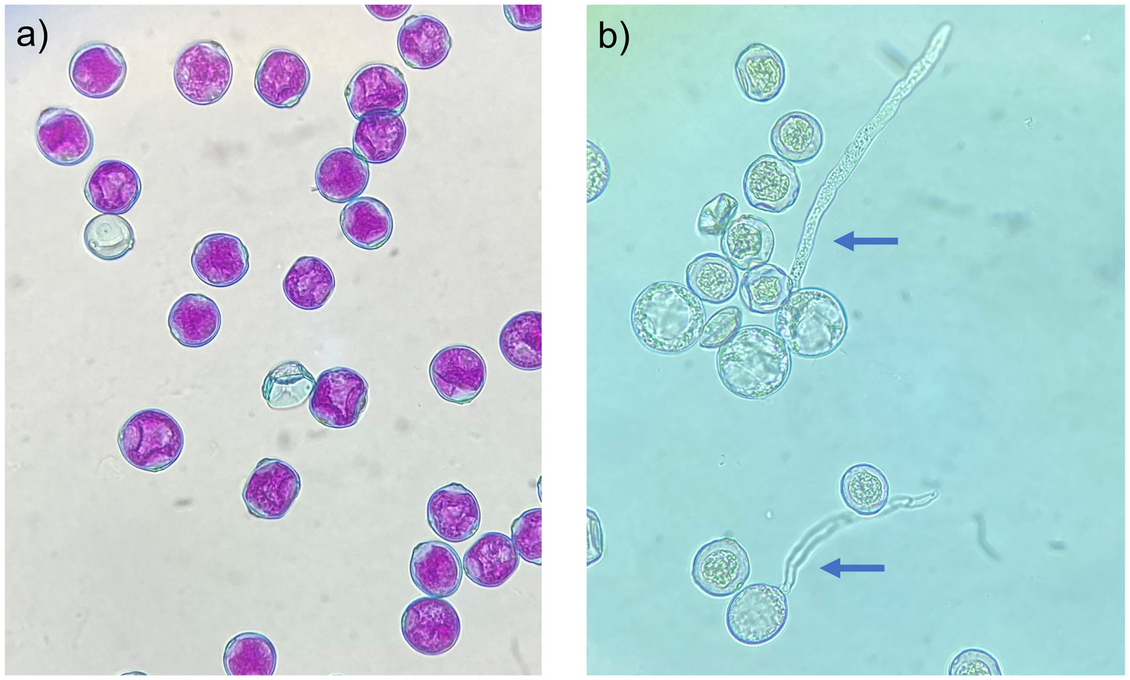
Fig 1. Differential staining and in vitro germination of Cannabis sativa pollen.
(a) Differential staining of non-aborted (pink cytoplasm with blue exines) and aborted pollen grains (blue exines), showing absorption of pink acid fuchsin in the cytoplasm of functional pollen grains. (b) In vitro germination of viable and inviable pollen grains, showing protrusion of the pollen tube in viable pollen grains (blue arrows).
PLOS ONE | https://doi.org/10.1371/journal.pone.0270799 July 7, 2022
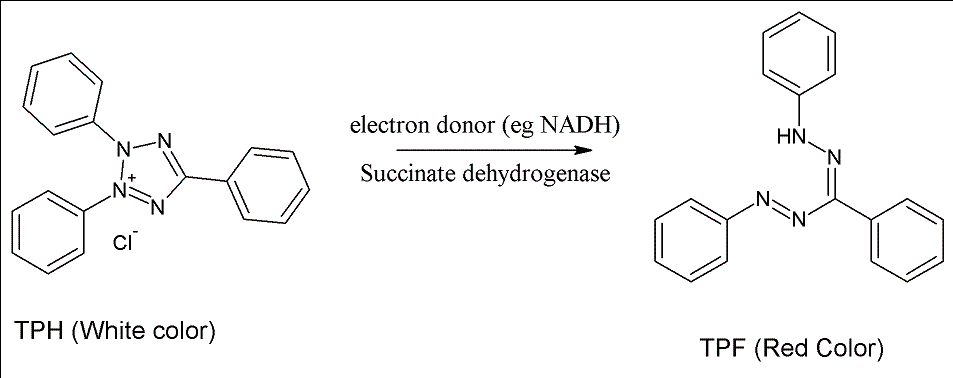
Tetrazolium Red is a colorless, water-soluble dye that is reduced to a deep red,
water-insoluble compound (formazan) mainly in the mitochondria of living cells,
predicted that all pollen grains would degrade at
38.3 weeks under room temperature conditions (Adj. R2 = 0.8903),
and
261.5 weeks under freezer conditions (Adj. R2 = 0.8991),
suggesting that long-term storage of pollen samples for genotyping is feasible.
Fig 1. Differential staining and in vitro germination of Cannabis sativa pollen.
(a) Differential staining of non-aborted (pink cytoplasm with blue exines) and aborted pollen grains (blue exines), showing absorption of pink acid fuchsin in the cytoplasm of functional pollen grains. (b) In vitro germination of viable and inviable pollen grains, showing protrusion of the pollen tube in viable pollen grains (blue arrows).
PLOS ONE | https://doi.org/10.1371/journal.pone.0270799 July 7, 2022
Tetrazolium Red is a colorless, water-soluble dye that is reduced to a deep red,
water-insoluble compound (formazan) mainly in the mitochondria of living cells,
Last edited:
acespicoli
Well-known member
acespicoli
Well-known member
simple fridge/freezer can
acespicoli
Well-known member
acespicoli
Well-known member
Dumigan, C.R.; Deyholos, M.K. Cannabis Seedlings Inherit Seed-Borne Bioactive and Anti-Fungal Endophytic Bacilli. Plants 2022, 11, 2127. https://doi.org/10.3390/plants11162127
All 15 accessions of Cannabis, including hemp and marijuana,
inherited seed-borne Paenibacillus mobilis with the capacity to solubilize mineral rock phosphate.
acespicoli
Well-known member
Abstract
As the industrial hemp (Cannabis sativa L.) market grows, there is a need for methods to clonally propagate parental breeding stock and new cultivars. Information is lacking on vegetative cutting propagation of hemp. We evaluated how propagation environment (intermittent mist vs. subirrigation under a humidity dome), indole-3-butyric acid (IBA) formulation (talc rooting powder vs. IBA in solution), and IBA concentration (0, 3000, or 8000 ppm) affected stem cuttings from ‘I3’, a cannabinoid-free cultivar of industrial hemp. Under mist or domes, rooting quality and percent declined at 8000 ppm IBA. Root and shoot quality and rooting percentage also were reduced in 3000 ppm IBA in solution treatment compared with talc. Our data show that for the cultivar tested, cuttings rooted at the highest percentage and produced the highest-quality roots and shoots with either no hormone or 3000 ppm talc powder. These treatments did equally well under humidity domes or intermittent mist.Keywords: Cannabis sativa; clonal propagation; humidity; IBA; intermittent mist; quick dip IBA; talc
The non-recreational hemp (Cannabis sativa L.) industry, particularly the cannabidiol sector, is expanding rapidly. Commercial growers and researchers are shifting to sterile triploids to avoid pollen contamination from male hemp plants grown nearby. As such, producers need reliable and efficient methods to clonally propagate industrial hemp on a large scale. Hemp is propagated either by seed (Potter, 2009), by stem cuttings (Caplan, 2018), or in vitro (Lata et al., 2017). Stem cuttings are a common method for propagation of hemp, but many factors affect the success and quality of the cuttings.
During vegetative propagation, transpiration is minimized by using methods like intermittent mist or humidity domes to maintain a high relative humidity around cuttings until they produce roots. If the relative humidity is too low, transpiration will be increased, which can cause cuttings to wilt faster and die (Owen, 2018). Humidity domes have previously been used in other experiments involving the propagation of woody plant species, such as aspen (Populus tremuloides Michx.) and balsam poplar (Populus balsamifera L.) (Wolken et al., 2010). Intermittent mist systems are the most common method of increasing humidity during cutting propagation. These systems apply short bursts of water in small droplets to the plants every few minutes throughout the duration of the light cycle. The water bursts help to keep humidity high and to minimize the rate of transpiration. Intermittent mist further reduces vapor pressure deficit by cooling the surface of the leaf via evaporation. Humidity domes (Campbell et al., 2019; Parsons et al., 2019) and intermittent mist systems (Clarke, 1981) have both been used in hemp propagation.
Indole-3-butyric acid (IBA) is often used for rooting in commercial operations (De Klerk et al., 1999) and is available in various formulations, concentrations, and application methods. IBA can be delivered to cuttings in talc or dissolved in alcohol to be used as a quick dip, whereas the potassium salt of IBA can be dissolved in water alone. Caplan (2018) found that a 0.2% (2000 ppm) IBA gel applied to hemp cuttings doubled the rooting percentage when compared with a 2000-ppm solution of willow (Salix sp.) extract. Although growers may opt to use talc or liquid formulations, it would be useful for growers to see how a single hemp breeding line reacts to each.
The purpose of our study was to evaluate the impact of environment (dome vs. intermittent mist), IBA formulation [talc vs. IBA/naphthalene acetic acid (NAA) quick dip], and IBA concentration on rooting percent and the root and shoot quality of stem cuttings from ‘I3’ hemp.
Materials and methods
Plant material
Stock plants were maintained in a greenhouse under a 24-h photoperiod with a mean canopy light intensity of 750 µmol·m−2·s−1 using 400-W high-pressure sodium lamps (Sun System, Vancouver, WA). Stock plants were potted at the beginning of Oct. 2020 as rooted cuttings in 5-gal containers. The containers were filled with a soilless potting mix (Metro-Mix; Sun Gro Horticulture, Agawam, MA) and perlite (Supreme Perlite Co., Portland, OR) (2:1 by volume)and incorporated with 67.5 g of 18N–2.6P–9.1K controlled-release fertilizer (Harrell’s, Lakeland, FL) per 2 ft3 of soilless potting mix (Metro-Mix). Plants were fertilized weekly with water-soluble 20N–8.7P–16.6K general-purpose fertilizer (Jack’s Professional; JR Peters, Allentown, PA) at 100-ppm concentration measured by a water-powered, non-electric chemical injector (Dosatron; Dosatron International, Clearwater, FL). The stock plants were 5 months old when cuttings were collected at the end of Feb. 2021. A mixture of terminal and subterminal cuttings was collected at 1400 hr. All cuttings were ≈4–5 inches in length, and each cutting had two or three fully expanded leaves. Terminal and subterminal cuttings were randomly assigned to each treatment combination. Cuttings were rooted in 10- by 20-inch plastic trays (Hydro Crunch, Walnut, CA) with drainage that contained soilless media (Sunshine Mix, Sun Gro Horticulture) and perlite (Supreme Perlite Co.) (2:1 by volume). Each drainage tray was set inside of a solid bottom 10-inch by 20-inch plastic tray (Hydro Crunch) that held water for subirrigation. The water in the bottom tray moved up through the medium by capillary action. Twenty-five cuttings were placed in each tray.Experimental design and environment
The experimental design was a randomized complete block design with a split plot arrangement of the environmental treatments. Each individual tray with 25 cuttings was counted as an experimental unit. A plastic-tented mist bench was used for the experiment. The bench was divided in half between the two environments (mist vs. no mist), and the remaining treatments were randomized within those two sub-plots. One half of the tented mist bench was equipped with mist emitters (CoolNet Pro Fogger; Netafim USA, Fresno, CA) suspended ≈30 inches above the bench surface, and the other half of the bench did not have any mist emitters; this half was used for the humidity domes. There were two IBA formulations and three different IBA concentrations applied to cuttings in both propagation methods. There were three replications for each of the 12 treatment combinations. Thirty-six different experimental units (trays) were evaluated, and a total of 900 cuttings were used. The IBA formulations were talc rooting powder (Hormex; Brooker Chemical Corp., Chatsworth, CA) and a mixture of 10,000 ppm IBA and 5000 ppm NAA in solution (Wood’s Rooting Compound; Earth Science Products Corp., Wilsonville, OR). Both IBA formulations were applied at 0, 3000, and 8000 ppm.Cuttings were collected, treated, and placed in the tented mist bench on 25 Feb. 2021. The tented mist bench was inside of a climate-controlled glass greenhouse with day/night set temperatures of 26/15 °C, with no supplemental lighting, and bottom heat at 22 °C. The trays were randomized within each section (mist vs. no mist). Trays in each group were watered at the time of propagation. From that point on, the humidity dome group was sub-irrigated with tap water as needed. The intermittent mist group was misted for 12 s once every 45 min from 0700 to 2000 hr. The humidity domes each had two vents on top. For days 1–5 after inserting cuttings into the growing medium, both vents were kept closed. On days 6–10, both vents were opened 25%. On days 11–15, both vents were opened 50%. On day 16, both vents were fully opened and remained open until the cuttings were harvested on day 28 (24 Mar. 2021).
Assessing root and shoot quality
Twenty-eight days after initiation, all cuttings were harvested, their root and shoot quality were assessed, and a rooting percentage was calculated. Root and shoot quality were assessed by rating the shoot and root system for each cutting on a scale of 0–4 (Fig. 1), with 4 being the best quality. A rating of 0 for shoots means that the shoot remained the same size as the initial cutting and did not produce any new leaves. A rating of 0 for roots means the cutting produced no roots. The mean root quality score and shoot quality score were calculated from the group of 25 cuttings within each experimental unit and subjected to analysis of variance (ANOVA) using RStudio (ver. 4.0.2; Allaire Corp., Newton, MA). ANOVA was used to compare results among the different propagation environments (humidity dome vs. intermittent mist), IBA formulations (talc powder vs. IBA in solution), and IBA concentrations. To independently assess the main effect treatments (different combinations of propagation environment, IBA formulation, and IBA concentration), Tukey’s honestly significant difference and Fisher’s least significant difference tests were performed.
Fig. 1.
Quality ratings used to evaluate (A) shoot quality and (B) root quality 4 weeks after treatment of ‘I3’ hemp cuttings. Quality was ranked on a 0–4 relative scale, with 4 representing the highest quality rating. A rating of 0 for shoots means that the shoot remained the same size as the initial cutting and did not produce any new leaves. A rating of 0 for roots means the cutting produced no roots.
Citation: HortTechnology 32, 3; 10.21273/HORTTECH05016-21
Results and discussion
Rooting success was high in both propagation environments, and there was no significant difference between them (P = 0.28); 88% of the cuttings propagated under the humidity domes rooted, and 84% of the cuttings under intermittent mist rooted (Table 1). However, there was a difference among all treatments (P = 0.094) if α was raised to 0.1. The propagation environment had no significant effect on root quality (Table 2). However, it had a significant, but modest, effect on shoot quality (Table 2). The average shoot quality rating for cuttings propagated under humidity domes was 1.85, and the average shoot quality rating for cuttings under intermittent mist was 1.62 [P < 0.01 (Fig. 2)]. Cuttings from the intermittent mist group were generally healthy, but mild amounts of chlorosis were observed on some leaves. Cuttings under the humidity domes did not show any signs of chlorosis. Other research has demonstrated that leaf chlorosis can occur under misting (Zhang and Graves, 1995).
Fig. 2.
A representation of the two-factor indole-3-butyric acid (IBA) formulation × IBA concentration interaction. Root and shoot quality are presented on a 0–4 relative scale for ‘I3’ hemp stem cuttings, with 4 being the best quality. A rating of 0 for shoots means that the shoot remained the same size as the initial cutting and did not produce any new leaves. A rating of 0 for roots means the cutting produced no roots. Ratings are separated by treatment combinations. The IBA concentration used in each treatment combination is stated below each treatment combination. “IBA in solution” represents the IBA/naphthalene acetic acid quick dip mixture. “Mist” refers to the intermittent mist propagation environment. “Dome” refers to the humidity dome propagation environment. “Talc” refers to talc based IBA powder. Shoot and root quality ratings are the two different response variables. Error bars represent standard error of the mean. Bars within each response variable (root or shoot quality) with the same letter are not different based on Tukey’s honest significant difference test (α = 0.05). Black letters refer to shoot quality; blue letters refer to root quality; 1 ppm = 1 mg·L−1.
Citation: HortTechnology 32, 3; 10.21273/HORTTECH05016-21
Impact of Indole-3-butyric Acid Concentration and Formulation and Propagation Environment on Rooting Success of ‘I3’ Hemp by Stem Cuttings
https://doi.org/10.21273/HORTTECH05016-21 1mg/liter = 1ppm
acespicoli
Well-known member
Your friend the freezer
A benevolent tool in our trade is the refrigerator and freezer. The fridge is extremely useful in extending the longevity of seed and pollen. The trick to successful freezing is to freeze deep (-10 to -40°F/-20 to -35°C) and then keep the seed undisturbed. Hard frozen objects are very fragile. The slightest shock may shatter crucial, delicate cell structures within the seed. Double wrap the seed in paper; little manilla envelopes work great.
I like to do small amounts, in one-time-use packets, to keep waste to a minimum. Then place the wrap into a plastic freezer bag, then place the freezer bag into a plastic tub or tupperware container. Now the seed is ready for the deep-freeze. In the fridge, storing seed in airtight, brown glass jars with a little rice or other non-toxic desiccant seems to work best.
I have had pollen last for years in a deep freeze. It must be frozen immediately after fresh collection from the plant, in as low a humidity as possible (preferably 0%). I like to shake the productive male flowers over a flat and clean piece of glass. The pollen pile is sifted to rid the unwanted plant material from the pure powder.
It is also useful to cut pollen with flour to stretch the amount. A pollen-to-flour ratio of 1:10 or even 1:100 works best. The cut pollen may then be separated into small, one-time-use amounts, stored in a flap of paper and frozen the same way as the seed. The frozen pollen must be applied to the live female flower immediately after thawing to increase viability.
(excerpt from DJ Short)
A benevolent tool in our trade is the refrigerator and freezer. The fridge is extremely useful in extending the longevity of seed and pollen. The trick to successful freezing is to freeze deep (-10 to -40°F/-20 to -35°C) and then keep the seed undisturbed. Hard frozen objects are very fragile. The slightest shock may shatter crucial, delicate cell structures within the seed. Double wrap the seed in paper; little manilla envelopes work great.
I like to do small amounts, in one-time-use packets, to keep waste to a minimum. Then place the wrap into a plastic freezer bag, then place the freezer bag into a plastic tub or tupperware container. Now the seed is ready for the deep-freeze. In the fridge, storing seed in airtight, brown glass jars with a little rice or other non-toxic desiccant seems to work best.
I have had pollen last for years in a deep freeze. It must be frozen immediately after fresh collection from the plant, in as low a humidity as possible (preferably 0%). I like to shake the productive male flowers over a flat and clean piece of glass. The pollen pile is sifted to rid the unwanted plant material from the pure powder.
It is also useful to cut pollen with flour to stretch the amount. A pollen-to-flour ratio of 1:10 or even 1:100 works best. The cut pollen may then be separated into small, one-time-use amounts, stored in a flap of paper and frozen the same way as the seed. The frozen pollen must be applied to the live female flower immediately after thawing to increase viability.
(excerpt from DJ Short)
acespicoli
Well-known member
Male isolation pollen chamber
acespicoli
Well-known member
@Roms photo of swazi seed https://www.icmag.com/threads/swazi-red-afropips.378343/
Very beautiful seed photo my friend, hope you enjoy seeing it posted here

>>>Best>ibes


