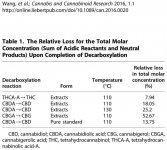
The results indicate complete conversion of THCA-A/D9-THC. The sum of the molar concentration of THCA-A and D9-THC decreased slightly after the decarboxylation, the relative loss is given in Table 1. Figure 4 also shows the data for CBN, which is a possible oxidation product of D9-THC. In this case, heating in the dark and in the absence of oxygen (vacuum oven) did not result in any significant oxidation of D9-THC to CBN. Finally, decarboxylation studies with pure THCA-A showed clearly that D9-THC was the only decomposition product observed after heating at 110°C for 40 min.
https://online.liebertpub.com/doi/pdfplus/10.1089/can.2016.0020
I attached a screenshot of Table 1 mentioned in the above quote.
Another statement from the paper,
The decarboxylation reaction for THCA-A was essentially stoichiometric with no side reactions. In particular, no CBN (a common oxidation by-product) was observed under the experimental conditions.
Another paper,
Decarboxylation of Δ 9-tetrahydrocannabinol: Kinetics and molecular modeling
https://www.researchgate.net/public...drocannabinol_Kinetics_and_molecular_modeling
2.1. Materials
Methanol was HPLC grade and was purchased from J.T. Baker (Deventer, The Netherlands). Medical grade cannabis plant mate- rial (female flower-tops) was obtained from Bureau Medicinale Cannabis (The Hague, The Netherlands). It had a D9-THCA content of about 18%, and virtually no free D9-THC. The water content was 3.6%. The standards of D9-THC (4.2 mg mL 1 in methanol – ref number 130-151205x) and D9-THCA (1.0 mg mL 1 – ref number 380-250407), with purity higher than 98%, were kindly donated by PRISNA B.V.
2.2. Method
A sample of around 400 mg cannabis was blended in a mixer, and heated at different temperatures in vacuum conditions for a certain time. The temperature range studied was from 90 to 140 °C. To follow the reaction rate, a sample was taken every 5 min for the first hour and then every half an hour until the conversion of D9-THCA to D9-THC was complete. Each solid sample was extracted with 50 mL methanol and sonicated for 15 min before being analysed with HPLC. In a series of extraction experiments it was determined that the extraction process was essentially complete. Calibration lines were determined for both D9-THCA and D9-THC. By this method the solid samples were inherently corrected for weight loss (up to 30% at 140 °C) during thermal treatment. Balances during the experiments, based on the molalities of D9-THCA and D9-THC, are >95%, indicating that the decarboxylation process itself proceeds with 100% selectivity. Some skeletal rearrangements however cannot be excluded.