Asprin is commonly used in conjunction with cannabis to synergistically enhance the pain killing qualities of both....
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>
'An Aspirin a Day' -- Just Another Cliché?
There was a time when only one brand of aspirin existed, and its manufacturer's 1920s ad campaign was intended to assure consumers that aspirin would not damage their hearts.
But we now know that aspirin can affect the heart. Today, aspirin is actually prescribed under its various generic and name brands for its heart-healthy effects.
"DOES NOT AFFECT THE HEART." That assurance in the Bayer aspirin ads of the 1920s spoke to concerns of the day that some drugs could damage the life-sustaining organ. Today it's clear that aspirin can affect the heart. Ironically, it turns out the effects are beneficial, so much so that some aspirin ads now carry the American Heart Association's seal to highlight the cardiovascular effects.
In fact, of the 80 million aspirin tablets Americans take each day, most are taken not for everyday aches and pains but to reduce the risk of heart disease, according to aspirin manufacturer Bayer Corp. (See "Aspirin's Other Uses.")
Based on studies showing aspirin's usefulness in treating cardiovascular disease, including heart attack and stroke, the Food and Drug Administration has approved its use to treat some of these serious conditions. Most recently, in 1998, FDA finalized a rule to give doctors updated information about the use of aspirin for men and women who have had a heart attack or stroke or are at high risk for them.
"Used the way it should be, the information should save a lot of lives," says Debra Bowen, M.D., deputy director of one of FDA's drug review offices.
"In addition," she says, "the information should reduce adverse reactions and allow doctors to better target those who need to use the product."
Beyond Pain Relief
As summarized in FDA's 1998 rule and in the updated professional labeling for aspirin, the 100-plus-year-old drug has been shown to reduce the risk of the following medical problems:
* stroke in those who have had a previous stroke or who have had a warning sign called a transient ischemic attack (mini-stroke)
* heart attack in those who have had a previous heart attack or experience angina (chest pain)
* death or complications from a heart attack if the drug is taken at the first signs of a heart attack
* recurrent blockage for those who have had heart bypass surgery or other procedures to clear blocked arteries, such as balloon angioplasty or carotid endarterectomy.
Under the rule, the recommended doses for cardiovascular uses are lower than those doctors had been prescribing since this new use became popular: generally, 50 to 325 milligrams once daily (75 to 325 milligrams for angina and previous heart attack).
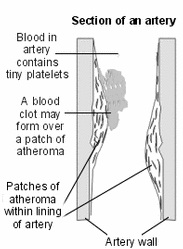
Scientists believe that aspirin's ability to reduce the body's production of hormone-like "prostaglandins" is the reason for both its effectiveness in relieving pain and reducing inflammation and its protective effects against heart attacks and strokes.
Prostaglandins, it seems, can cause platelets in the blood to stick together, which can eventually lead to blocked blood vessels and can prevent delivery of oxygen-rich blood to the tissues.
"When a clot forms in the brain, it can cause a stroke, and in the heart, a heart attack," explains George Sopko, M.D., the head of the Interventional Cardiology Scientific Research Group at the National Institutes of Health. Reduce the prostaglandins, and you reduce the risk of dangerous blood clots, heart attacks, and strokes.
"Aspirin is a great drug: effective, cheap, and relatively safe," Sopko says. "The drug has been used by just about everybody, so it may not have the sex appeal of newer drugs, but it can have a huge beneficial impact if used properly. Looking at aspirin's impact, on heart attacks for example, it may be equal to or better than some drug therapies that cost thousands of dollars."
Other pain relievers and fever-reducing drugs, such as acetaminophen, ibuprofen, naproxyn sodium, and ketoprofen, have not been shown to have aspirin's beneficial impact on cardiovascular health.
"It's not the pain-relieving quality that is the major thrust of aspirin's beneficial cardiovascular effects," Sopko explains, "but its pharmacological effect on platelets."
Not for Everyone
Although aspirin is a familiar and readily available drug, people shouldn't take it for its cardiovascular benefits without discussing the risks of long-term use with a doctor, cautions Charles H. Hennekens, M.D., chief of preventive medicine at Brigham and Women's Hospital in Boston. "If someone feels they're a candidate, they should talk to their doctor in making the judgment if the benefits outweigh the risks."
The same quality that gives aspirin its potential benefit--its ability to inhibit clotting of the blood--may increase the risk of excessive bleeding, including the possibility of bleeding in the brain. Some other possible risks are:
* Stomach irritation. Aspirin can irritate the stomach lining and cause heartburn, pain, nausea, vomiting, and, over time, more serious consequences such as internal bleeding, ulcers, and holes in the stomach or intestines. Chronic alcohol users may be at increased risk of stomach bleeding, as well as liver damage, from aspirin use.
* Ringing in the ears. At high doses, aspirin may cause temporary ringing in the ears and hearing loss, which usually disappear when the dose is lowered.
* Allergy. Facial swelling and sometimes an asthma attack may occur in the two out of 1,000 people who are allergic to aspirin, according to the Mayo Clinic in Rochester, Minn.
* In children, Reye syndrome. While not a problem among candidates for cardiovascular aspirin use, aspirin should not be used for children's flu-like symptoms or chickenpox because of the risk of this rare but serious disease.
Because of its risks, aspirin is not approved for decreasing the risk of heart attack in healthy individuals.
Even Hennekens isn't ready to recommend an aspirin a day for everyone, although he headed up the celebrated 1988 "Physicians' Health Study," which showed aspirin's protective effects in healthy people.
Why can't this so-called "wonder drug" help everyone?
Hennekens' example: A 30-year-old woman's risk of a heart attack is typically "very small," even over the next 30 years. "It would be unfortunate if such a young woman was taking aspirin," he explains, "because it would give no benefit and could cause gastrointestinal effects or dangerous bleeding."
Head Start
In the wide range of patients who could see large benefits, aspirin, regrettably, is not used nearly enough, according to Hennekens.
Studies bear this out, including a 1998 survey of elderly heart attack survivors entering nursing homes, which found that fewer than one in five were taking aspirin.
According to the American Heart Association, 5,000 to 10,000 of the 900,000 lives lost each year to cardiovascular disease could be saved if more people took aspirin upon the first signs of a heart attack.
Some typical signs are an uncomfortable pressure or pain in the center of the chest (sometimes along with lightheadedness, fainting, shortness of breath, nausea, or sweating) or a pain going to the shoulders, neck and arms.
Aspirin should be used by "just about everyone" who has survived a heart attack or stroke due to a blocked blood vessel, Hennekens emphasizes, or who within the previous 24 hours has had symptoms of an evolving heart attack.
While appropriate aspirin use is important, experts say it is by no means a cure-all.
"In the time crunch surrounding a heart attack, taking an aspirin provides you a head-start therapy and a better chance for a good outcome," Sopko says. "But it should never be a substitute for a physician's attention."
And aspirin should not replace a healthy lifestyle or other helpful medical steps, FDA's Bowen says.
"Physicians really need to look at aspirin in the context of complete care, as part of a whole treatment plan for people at risk of heart attack or stroke."
Aspirin's Other Uses
Aspirin is sometimes used to treat rheumatoid arthritis, juvenile rheumatoid arthritis, osteoarthritis, and some other rheumatological diseases.
Aspirin labeling was updated in 1998, and now provides information on specific dosing, side effects, and toxicity of aspirin for these conditions.
More potential medical uses for aspirin are still under study--everything from treating migraines and colon, ovarian and breast cancer to improving brain function.
Could an aspirin a day help you retain your memory as you age by preventing clogging of the arteries in the brain? It remains to be proven, but early studies suggest it's possible.
Three Drinks = No Pain Relievers
Aspirin and all other over-the-counter pain relievers and fever reducers for adults will soon carry a warning to people who drink three or more alcoholic beverages a day: Talk with your doctor before using these drugs. Heavy drinkers may have an increased risk of liver damage and stomach bleeding from these medicines, which contain aspirin, other salicylates, acetaminophen, ibuprofen, naproxen sodium, or ketoprofen.
The alcohol warning is required under an FDA rule (distinct from the aspirin labeling rule), which was finalized in 1998 and gives manufacturers some time to make the label changes. Some newer over-the-counter pain relievers, including Aleve (naproxyn sodium), Orudis KT and Actron (ketoprofen), Advil Liquigels (solubilized ibuprofen), and Tylenol Extended Release (acetaminophen), have already been required to carry a warning for heavy drinkers but were not required to include the specific risks. These products, too, will need to comply with the 1998 rule.
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>
For More Information on Aspirin and the Heart:
American Heart Association
http://www.americanheart.org/presenter.jhtml?identifier=1200000
Aspirin Foundation of America
http://www.aspirin.org/
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>
'An Aspirin a Day' -- Just Another Cliché?
There was a time when only one brand of aspirin existed, and its manufacturer's 1920s ad campaign was intended to assure consumers that aspirin would not damage their hearts.
But we now know that aspirin can affect the heart. Today, aspirin is actually prescribed under its various generic and name brands for its heart-healthy effects.
"DOES NOT AFFECT THE HEART." That assurance in the Bayer aspirin ads of the 1920s spoke to concerns of the day that some drugs could damage the life-sustaining organ. Today it's clear that aspirin can affect the heart. Ironically, it turns out the effects are beneficial, so much so that some aspirin ads now carry the American Heart Association's seal to highlight the cardiovascular effects.
In fact, of the 80 million aspirin tablets Americans take each day, most are taken not for everyday aches and pains but to reduce the risk of heart disease, according to aspirin manufacturer Bayer Corp. (See "Aspirin's Other Uses.")
Based on studies showing aspirin's usefulness in treating cardiovascular disease, including heart attack and stroke, the Food and Drug Administration has approved its use to treat some of these serious conditions. Most recently, in 1998, FDA finalized a rule to give doctors updated information about the use of aspirin for men and women who have had a heart attack or stroke or are at high risk for them.
"Used the way it should be, the information should save a lot of lives," says Debra Bowen, M.D., deputy director of one of FDA's drug review offices.
"In addition," she says, "the information should reduce adverse reactions and allow doctors to better target those who need to use the product."
Beyond Pain Relief
As summarized in FDA's 1998 rule and in the updated professional labeling for aspirin, the 100-plus-year-old drug has been shown to reduce the risk of the following medical problems:
* stroke in those who have had a previous stroke or who have had a warning sign called a transient ischemic attack (mini-stroke)
* heart attack in those who have had a previous heart attack or experience angina (chest pain)
* death or complications from a heart attack if the drug is taken at the first signs of a heart attack
* recurrent blockage for those who have had heart bypass surgery or other procedures to clear blocked arteries, such as balloon angioplasty or carotid endarterectomy.
Under the rule, the recommended doses for cardiovascular uses are lower than those doctors had been prescribing since this new use became popular: generally, 50 to 325 milligrams once daily (75 to 325 milligrams for angina and previous heart attack).
Scientists believe that aspirin's ability to reduce the body's production of hormone-like "prostaglandins" is the reason for both its effectiveness in relieving pain and reducing inflammation and its protective effects against heart attacks and strokes.
Prostaglandins, it seems, can cause platelets in the blood to stick together, which can eventually lead to blocked blood vessels and can prevent delivery of oxygen-rich blood to the tissues.
"When a clot forms in the brain, it can cause a stroke, and in the heart, a heart attack," explains George Sopko, M.D., the head of the Interventional Cardiology Scientific Research Group at the National Institutes of Health. Reduce the prostaglandins, and you reduce the risk of dangerous blood clots, heart attacks, and strokes.
"Aspirin is a great drug: effective, cheap, and relatively safe," Sopko says. "The drug has been used by just about everybody, so it may not have the sex appeal of newer drugs, but it can have a huge beneficial impact if used properly. Looking at aspirin's impact, on heart attacks for example, it may be equal to or better than some drug therapies that cost thousands of dollars."
Other pain relievers and fever-reducing drugs, such as acetaminophen, ibuprofen, naproxyn sodium, and ketoprofen, have not been shown to have aspirin's beneficial impact on cardiovascular health.
"It's not the pain-relieving quality that is the major thrust of aspirin's beneficial cardiovascular effects," Sopko explains, "but its pharmacological effect on platelets."
Not for Everyone
Although aspirin is a familiar and readily available drug, people shouldn't take it for its cardiovascular benefits without discussing the risks of long-term use with a doctor, cautions Charles H. Hennekens, M.D., chief of preventive medicine at Brigham and Women's Hospital in Boston. "If someone feels they're a candidate, they should talk to their doctor in making the judgment if the benefits outweigh the risks."
The same quality that gives aspirin its potential benefit--its ability to inhibit clotting of the blood--may increase the risk of excessive bleeding, including the possibility of bleeding in the brain. Some other possible risks are:
* Stomach irritation. Aspirin can irritate the stomach lining and cause heartburn, pain, nausea, vomiting, and, over time, more serious consequences such as internal bleeding, ulcers, and holes in the stomach or intestines. Chronic alcohol users may be at increased risk of stomach bleeding, as well as liver damage, from aspirin use.
* Ringing in the ears. At high doses, aspirin may cause temporary ringing in the ears and hearing loss, which usually disappear when the dose is lowered.
* Allergy. Facial swelling and sometimes an asthma attack may occur in the two out of 1,000 people who are allergic to aspirin, according to the Mayo Clinic in Rochester, Minn.
* In children, Reye syndrome. While not a problem among candidates for cardiovascular aspirin use, aspirin should not be used for children's flu-like symptoms or chickenpox because of the risk of this rare but serious disease.
Because of its risks, aspirin is not approved for decreasing the risk of heart attack in healthy individuals.
Even Hennekens isn't ready to recommend an aspirin a day for everyone, although he headed up the celebrated 1988 "Physicians' Health Study," which showed aspirin's protective effects in healthy people.
Why can't this so-called "wonder drug" help everyone?
Hennekens' example: A 30-year-old woman's risk of a heart attack is typically "very small," even over the next 30 years. "It would be unfortunate if such a young woman was taking aspirin," he explains, "because it would give no benefit and could cause gastrointestinal effects or dangerous bleeding."
Head Start
In the wide range of patients who could see large benefits, aspirin, regrettably, is not used nearly enough, according to Hennekens.
Studies bear this out, including a 1998 survey of elderly heart attack survivors entering nursing homes, which found that fewer than one in five were taking aspirin.
According to the American Heart Association, 5,000 to 10,000 of the 900,000 lives lost each year to cardiovascular disease could be saved if more people took aspirin upon the first signs of a heart attack.
Some typical signs are an uncomfortable pressure or pain in the center of the chest (sometimes along with lightheadedness, fainting, shortness of breath, nausea, or sweating) or a pain going to the shoulders, neck and arms.
Aspirin should be used by "just about everyone" who has survived a heart attack or stroke due to a blocked blood vessel, Hennekens emphasizes, or who within the previous 24 hours has had symptoms of an evolving heart attack.

While appropriate aspirin use is important, experts say it is by no means a cure-all.
"In the time crunch surrounding a heart attack, taking an aspirin provides you a head-start therapy and a better chance for a good outcome," Sopko says. "But it should never be a substitute for a physician's attention."
And aspirin should not replace a healthy lifestyle or other helpful medical steps, FDA's Bowen says.
"Physicians really need to look at aspirin in the context of complete care, as part of a whole treatment plan for people at risk of heart attack or stroke."
Aspirin's Other Uses
Aspirin is sometimes used to treat rheumatoid arthritis, juvenile rheumatoid arthritis, osteoarthritis, and some other rheumatological diseases.
Aspirin labeling was updated in 1998, and now provides information on specific dosing, side effects, and toxicity of aspirin for these conditions.
More potential medical uses for aspirin are still under study--everything from treating migraines and colon, ovarian and breast cancer to improving brain function.
Could an aspirin a day help you retain your memory as you age by preventing clogging of the arteries in the brain? It remains to be proven, but early studies suggest it's possible.
Three Drinks = No Pain Relievers
Aspirin and all other over-the-counter pain relievers and fever reducers for adults will soon carry a warning to people who drink three or more alcoholic beverages a day: Talk with your doctor before using these drugs. Heavy drinkers may have an increased risk of liver damage and stomach bleeding from these medicines, which contain aspirin, other salicylates, acetaminophen, ibuprofen, naproxen sodium, or ketoprofen.
The alcohol warning is required under an FDA rule (distinct from the aspirin labeling rule), which was finalized in 1998 and gives manufacturers some time to make the label changes. Some newer over-the-counter pain relievers, including Aleve (naproxyn sodium), Orudis KT and Actron (ketoprofen), Advil Liquigels (solubilized ibuprofen), and Tylenol Extended Release (acetaminophen), have already been required to carry a warning for heavy drinkers but were not required to include the specific risks. These products, too, will need to comply with the 1998 rule.
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>
For More Information on Aspirin and the Heart:
American Heart Association
http://www.americanheart.org/presenter.jhtml?identifier=1200000
Aspirin Foundation of America
http://www.aspirin.org/
Last edited:


