CrushnYuba
Well-known member
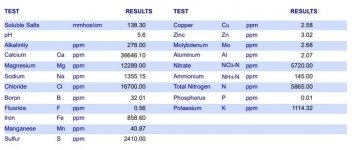
I know there have already been threads on this. I'm trying to make a diy calmag plus. I have calcium nitrate kicking around. I don't have magnesium nitrate and I'm having trouble finding it. Don't want to use epsom.
The only mag nitrate i can find has allot of magnesium oxide in it as well. Is this available to plants? Do i account for the mag oxide in my ratios?
The only mag nitrate i can find has allot of magnesium oxide in it as well. Is this available to plants? Do i account for the mag oxide in my ratios?