dizzlekush
Member
Oh I almost forgot to mention I have a sealed room and am using C02 so I added the NH4 into the mix. Only costs .60 per 10 gallons if you order salts from customhydronutrients.com and JRpeters.
Per Gallon
Jacks (5-12-26)-2g
AgSil14H- 1.2g
Magnesium Sulfate- .9g
Ammonium Sulfate-.52g
Iron DPTA 10%- .02g
Makes:
1.6mS/cm
N03-128.242ppm
NH4-29.126ppm
P-26.668ppm
K-198.237ppm
Ca-145.237ppm
Mg-56.834ppm
S-64.267ppm
Fe-2.1ppm
B-.264ppm
Cu-.079ppm
Mo-.053ppm
Mn-.264ppm
Si-78.401ppm
Hey buddy. COCO!?!?
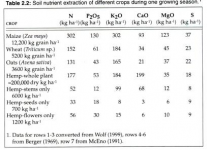
Im guessing you left CalciNit out of your weight/gallon section. About your ppms:
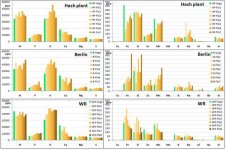
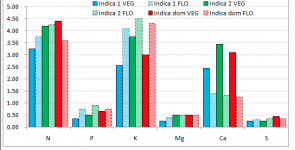
I think your NO3:NH4 ratio is a bit off. i know you're copying Spurr's profile which was meant for creating a pH stable solution from RO water. Spurr's 4:1 ratio is for pH stability, he even admits that a 10:1 - 20:1 ratio is more optimal for cannabis growth. The max ppm of NH4 you will ever need is ~15ppm for optimal cannabis growth, even in CO2 rich environments.
All of your micro's except for Fe are too low. Drop the FeDPTA and keep adding Jacks til your Mn and B are over/at 0.4ppm. you'll prolly need to lower potassium silicate to not keep K too high. don't worry about that. Si as low as 25ppm is shown to be beneficial in studies.
I just remembered you said this was for vegetative growth. I dont believe in higher K than N in vegetative growth, at max k should be equal. This means dropping the Magnesium sulfate and using Magnesium nitrate instead for veg. No need for so much S in veg. Usually plants need Mg, S, and P in similar amounts. Cannabis seems to follow that rule, at least in vegetative growth.