dizzlekush
Member
This is a thread for users of and those curious about the products Jacks Professional Hydro 5-12-26, Peters Hydrosol 5-11-26 and all other products that are:
Product A: Potassium Nitrate, (some variety of) Potassium Phosphate, Magnesium Sulfate and a micro-nutrient package
Product B: Calcium Nitrate, which is actually almost always the hydrated double salt Calcium Ammonium Nitrate Decahydrate [5Ca(NO3)2.NH4NO3.10H2O]
These products have been gaining popularity quickly due to the unbeatable low price, ease of use, flexibility and quality results. Sharing of formulations, experiences, pictures, questions regarding how to use such products, do-it-yourselfers and the like are all more than welcome here. This thread will also attempt to delve into the nutritional requirements for optimal cannabis growth and the science behind properly meeting those requirements.
Where such products can be sourced online:
Peter's Professional Hydrosol
http://customhydronutrients.com/zencart/index.php?main_page=index&cPath=1_43_40
Jack's Professional Hydro
http://www.jrpeters.com/Products/Hydroponics/Buy-Hydroponics.html
Hydro-Gardens Mixes
http://www.hydro-gardens.com/51126.htm
Verti-Gro Mixes
http://vertigro.com/products/fertilizers.php
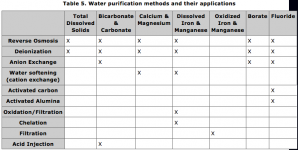
Although Hydrosol is the name of the specific product made by Scotts, I shall use the name to refer to ALL products of the same nature. One thing worth mentioning is that the Hydrosol provided by Jack's is formulated with extra MgSO4 and will NOT need epsom salts added in most situations, if water Mg levels are high a different Hydrosol (such as Peters by Scotts) should be used. Such products are most commonly used in cannabis cultivations by combining the Hydrosol:CaNO3 at a simple 3:2 (i.e.1:0.666) ratio. Your ability to tweak your formulations can improve by purchasing other pure salts to combine with your Hydrosol/CaNO3 'base' formula. One word of caution about buying pure salts is always source all salts containing P from sources that have had legitimate testing of heavy metals as a concern for ALL P sources is leftover amounts of radioactive lead and polonium. Here's where other nutrients can be sourced in their pure form (your CaNO3 can be purchased from any of these sources as well):
J.R. Peters Inc.
http://www.jrpeters.com/Products/Specialty-Nutrients/Buy-Specialty-Nutrients.html
Custom Hydro Nutrients
http://customhydronutrients.com/zencart/index.php?main_page=index&cPath=1
Hydro-Gardens
http://www.hydro-gardens.com/fertcomp.htm
Other chemicals i personally suggest for Hydrosol/CaNO3 formulations are:
Potassium Silicate (AgSil 16H has the best K:Si ratio)
Ammonium Sulfate and/or Diammonium Phosphate (NH4 is necessary when CO2 boosting & having too significant a NO3:NH4 ratio can lead to pH issues)
Magnesium Sulfate heptahydrate
Magnesium Nitrate hexahydrate (Alternative N source for vegetative formulations, Ammonium Nitrate would be better)
Citric acid and/or Sulfuric and/or Phosophoric acid for pH down
Potassium Carbonate for pH up. (Works especially well with Phosphoric acid to buffer pH)
Tools to assist in formulating nutrient profiles:
HydroBuddy Nutrient Calculator (download only)
http://scienceinhydroponics.com/
ManicBotanix Nutrient Calculator (no download necessary)
http://www.manicbotanix.com/calculators/ppm-in-solution-calc.php
ManicBotanix Chemical Formula & Mole Calculator (good for quick pure salt calculations)
http://www.manicbotanix.com/calculators/molecalc.php
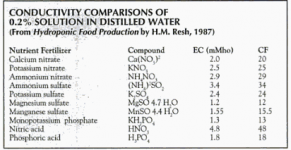
The HydroBuddy nutrient calculator is the most comprehensive free nutrient calculator by far with many perks that other calculators don't have. It is definitely worth downloading. If you like it, don't forget to send Daniel a donation for providing his work for free. When it comes to online calculators, the ones made by ManicBotanix are the best i've seen.
Product A: Potassium Nitrate, (some variety of) Potassium Phosphate, Magnesium Sulfate and a micro-nutrient package
Product B: Calcium Nitrate, which is actually almost always the hydrated double salt Calcium Ammonium Nitrate Decahydrate [5Ca(NO3)2.NH4NO3.10H2O]
These products have been gaining popularity quickly due to the unbeatable low price, ease of use, flexibility and quality results. Sharing of formulations, experiences, pictures, questions regarding how to use such products, do-it-yourselfers and the like are all more than welcome here. This thread will also attempt to delve into the nutritional requirements for optimal cannabis growth and the science behind properly meeting those requirements.
Where such products can be sourced online:
Peter's Professional Hydrosol
http://customhydronutrients.com/zencart/index.php?main_page=index&cPath=1_43_40
Jack's Professional Hydro
http://www.jrpeters.com/Products/Hydroponics/Buy-Hydroponics.html
Hydro-Gardens Mixes
http://www.hydro-gardens.com/51126.htm
Verti-Gro Mixes
http://vertigro.com/products/fertilizers.php
Although Hydrosol is the name of the specific product made by Scotts, I shall use the name to refer to ALL products of the same nature. One thing worth mentioning is that the Hydrosol provided by Jack's is formulated with extra MgSO4 and will NOT need epsom salts added in most situations, if water Mg levels are high a different Hydrosol (such as Peters by Scotts) should be used. Such products are most commonly used in cannabis cultivations by combining the Hydrosol:CaNO3 at a simple 3:2 (i.e.1:0.666) ratio. Your ability to tweak your formulations can improve by purchasing other pure salts to combine with your Hydrosol/CaNO3 'base' formula. One word of caution about buying pure salts is always source all salts containing P from sources that have had legitimate testing of heavy metals as a concern for ALL P sources is leftover amounts of radioactive lead and polonium. Here's where other nutrients can be sourced in their pure form (your CaNO3 can be purchased from any of these sources as well):
J.R. Peters Inc.
http://www.jrpeters.com/Products/Specialty-Nutrients/Buy-Specialty-Nutrients.html
Custom Hydro Nutrients
http://customhydronutrients.com/zencart/index.php?main_page=index&cPath=1
Hydro-Gardens
http://www.hydro-gardens.com/fertcomp.htm
Other chemicals i personally suggest for Hydrosol/CaNO3 formulations are:
Potassium Silicate (AgSil 16H has the best K:Si ratio)
Ammonium Sulfate and/or Diammonium Phosphate (NH4 is necessary when CO2 boosting & having too significant a NO3:NH4 ratio can lead to pH issues)
Magnesium Sulfate heptahydrate
Magnesium Nitrate hexahydrate (Alternative N source for vegetative formulations, Ammonium Nitrate would be better)
Citric acid and/or Sulfuric and/or Phosophoric acid for pH down
Potassium Carbonate for pH up. (Works especially well with Phosphoric acid to buffer pH)
Tools to assist in formulating nutrient profiles:
HydroBuddy Nutrient Calculator (download only)
http://scienceinhydroponics.com/
ManicBotanix Nutrient Calculator (no download necessary)
http://www.manicbotanix.com/calculators/ppm-in-solution-calc.php
ManicBotanix Chemical Formula & Mole Calculator (good for quick pure salt calculations)
http://www.manicbotanix.com/calculators/molecalc.php
The HydroBuddy nutrient calculator is the most comprehensive free nutrient calculator by far with many perks that other calculators don't have. It is definitely worth downloading. If you like it, don't forget to send Daniel a donation for providing his work for free. When it comes to online calculators, the ones made by ManicBotanix are the best i've seen.