Bubbleblower
Member
In reality CBD oil doesn't work any better than a placebo and there is a large risk it makes the problems even worse.
Highlights from this study from the Department of Pediatrics and Neurology, Children's Hospital Colorado and the University of Colorado, Anschutz Medical Campus:
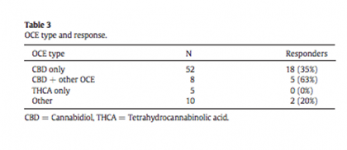
•Parents in our study reported a 33% response to OCE*.
•The response rate is similar to previously reported placebo response rates.
•Reported response was not correlated with improved background EEG data.
Adverse events (AEs) occurred in 47% of patients with increased seizures (transient or persistent) or new seizures in 21%.
* There was a huge bias, parents that moved to CO to get OCE reported a 47% response but parents originally from CO only a 22% response.
Highlights from this study from the Department of Pediatrics and Neurology, Children's Hospital Colorado and the University of Colorado, Anschutz Medical Campus:
•Parents in our study reported a 33% response to OCE*.
•The response rate is similar to previously reported placebo response rates.
•Reported response was not correlated with improved background EEG data.
Adverse events (AEs) occurred in 47% of patients with increased seizures (transient or persistent) or new seizures in 21%.
* There was a huge bias, parents that moved to CO to get OCE reported a 47% response but parents originally from CO only a 22% response.