Chonkski
Member
Also: I think it might be wise for those of us who wish to explore steam distillation to start a new thread
And so here we are!
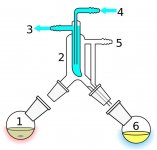
Anyways, for those of you wondering. I am very curious to start experimenting with refining polished extract even further. Being able to put the title of 'solvent free' and achieving percentages of the 80's and up total cannabinoid, out of what was once poopy outdoor black goo bho.
I know this is becoming very popular, mostly on the consumer side. Which makes finding out specifics of the process a bit harder to find, many are trying to hide/cover up processes as well.
So this may be the forum where all of that can come to light! Then we can go ahead and rush towards the next big thing, lol.
I am no bio chemist major, so any help on the process would be greatly appreciated. I just have a lot of time and material to experiment with so I will document how my experiments go here.
Hope others share the same interest as I!
CK