PCBuds
Well-known member
I watered my plant again with the RO water solution and tested the reservoir and got this...

It's even higher than yesterday, so I siphoned the solution out, PH'ed it down to this and poured it back down at the stalk.
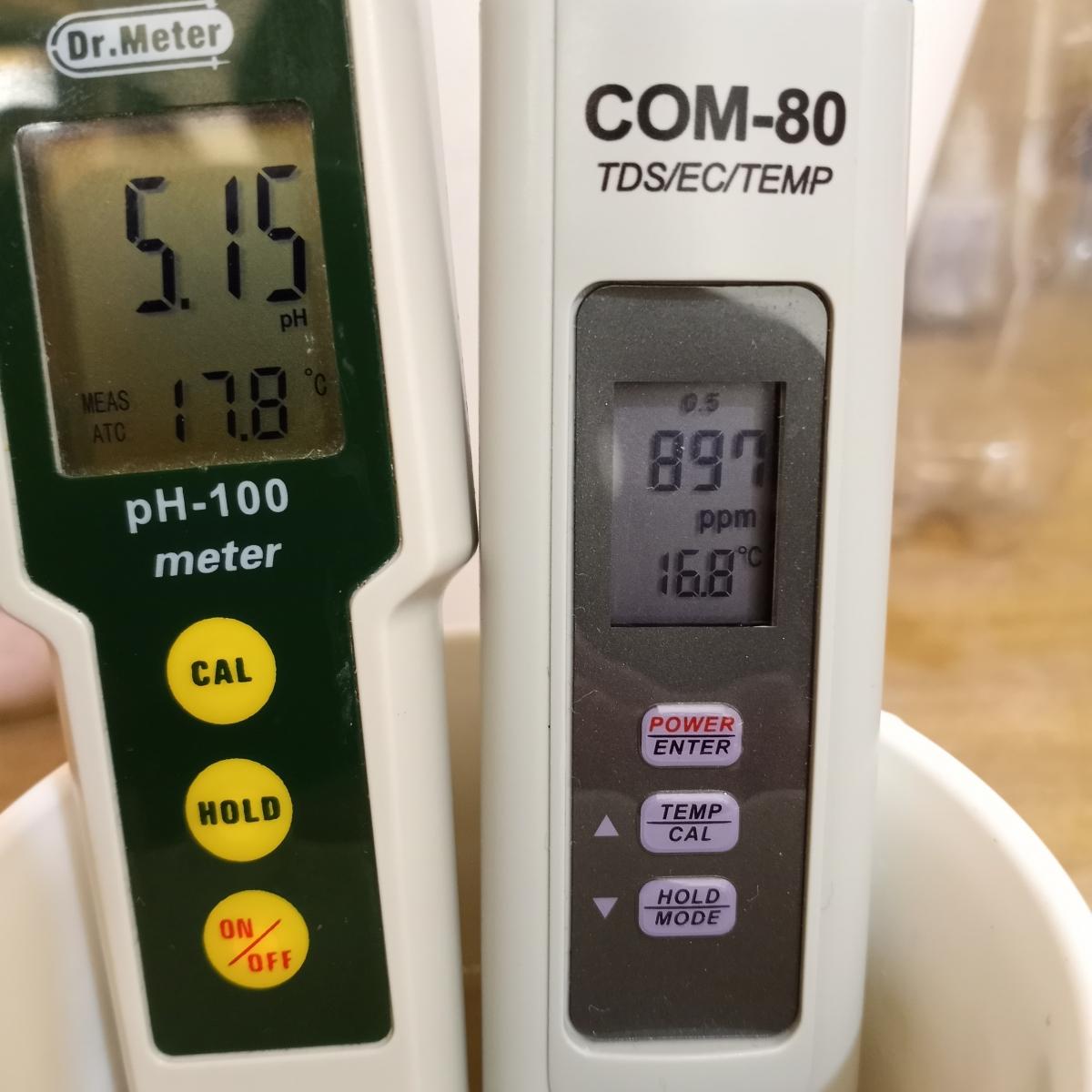
I tested the reservoir again and got this.

So I siphoned it off again and PH'ed it down again.

I ended up with this as my PH in the reservoir...

I figure that's good enough for now, especially since the plant looks fine to me anyway.
I'm wondering if it's a case of "If it ain't broke, don't fix it"?
I make shock the plant or upset my bennies by doing it? I dunno?
I'll check it again tomorrow to see if the PH has risen and maybe feed in some more solution PH'ed low again.
It's even higher than yesterday, so I siphoned the solution out, PH'ed it down to this and poured it back down at the stalk.
I tested the reservoir again and got this.
So I siphoned it off again and PH'ed it down again.
I ended up with this as my PH in the reservoir...
I figure that's good enough for now, especially since the plant looks fine to me anyway.
I'm wondering if it's a case of "If it ain't broke, don't fix it"?
I make shock the plant or upset my bennies by doing it? I dunno?
I'll check it again tomorrow to see if the PH has risen and maybe feed in some more solution PH'ed low again.




