Hookahhead
Active member
Success!
Success!
They say seeing is believing. Today I slipped into the lab over my lunch break to use the microscope. This scope is equipped with a camera, but the laptop that hooks up to it was being used. So I took the pictures using my cellphone over the eyepiece. I used the first successful bottle (white cap @ 28 days) for my sample.
Here’s what we’re looking for
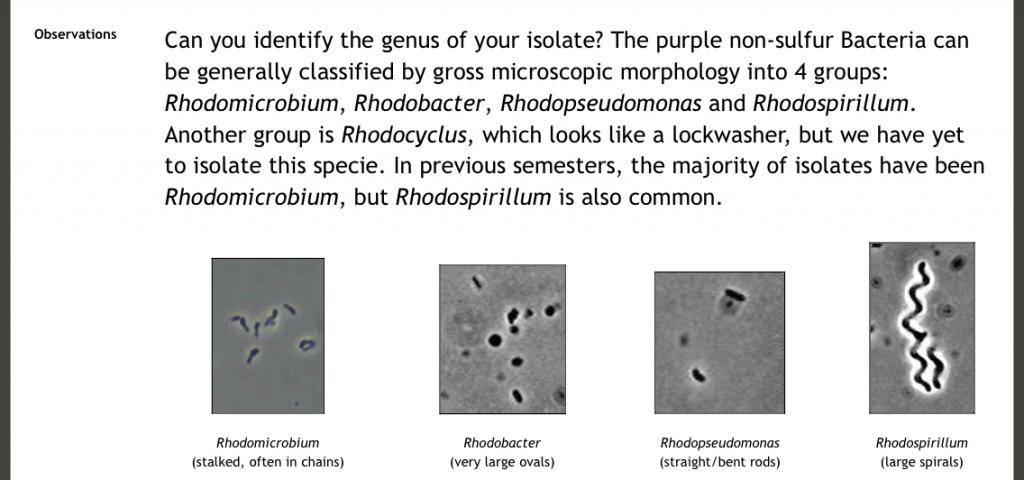
Source: https://www.mbio.ncsu.edu/mb452/purple_nonsulfurs/purples.html
What did I find? A ton of life!
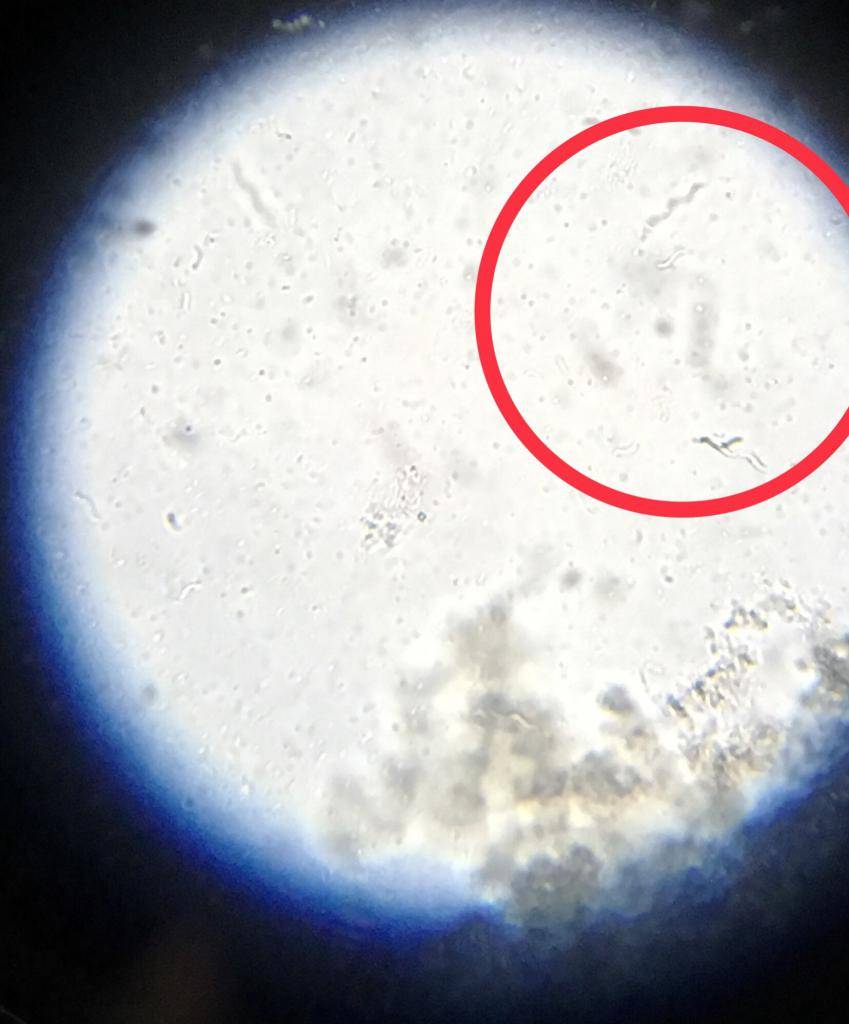
Rhodospirillum - Live
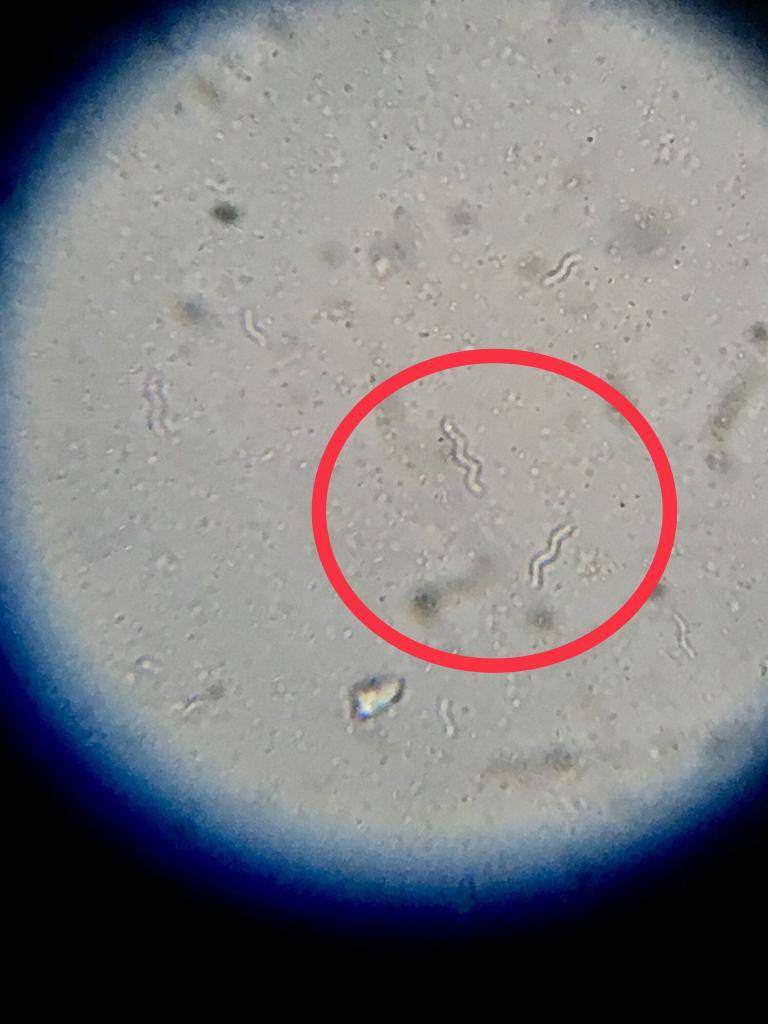
Rhodospirillum - Dead
Rhodospirillum is the easiest to identify because they’re large and have a unique shape. I’m hoping Microbeman can chime in and confirm/deny these others. Again, microscopy is not my forte.
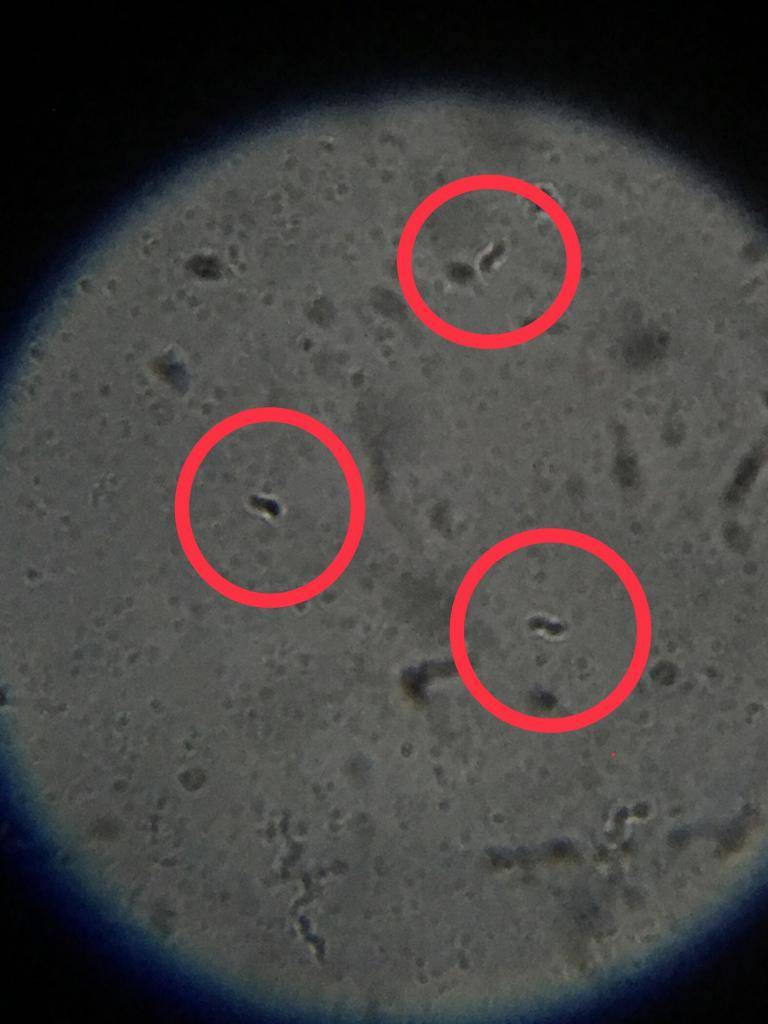
Rhodomicrobium?
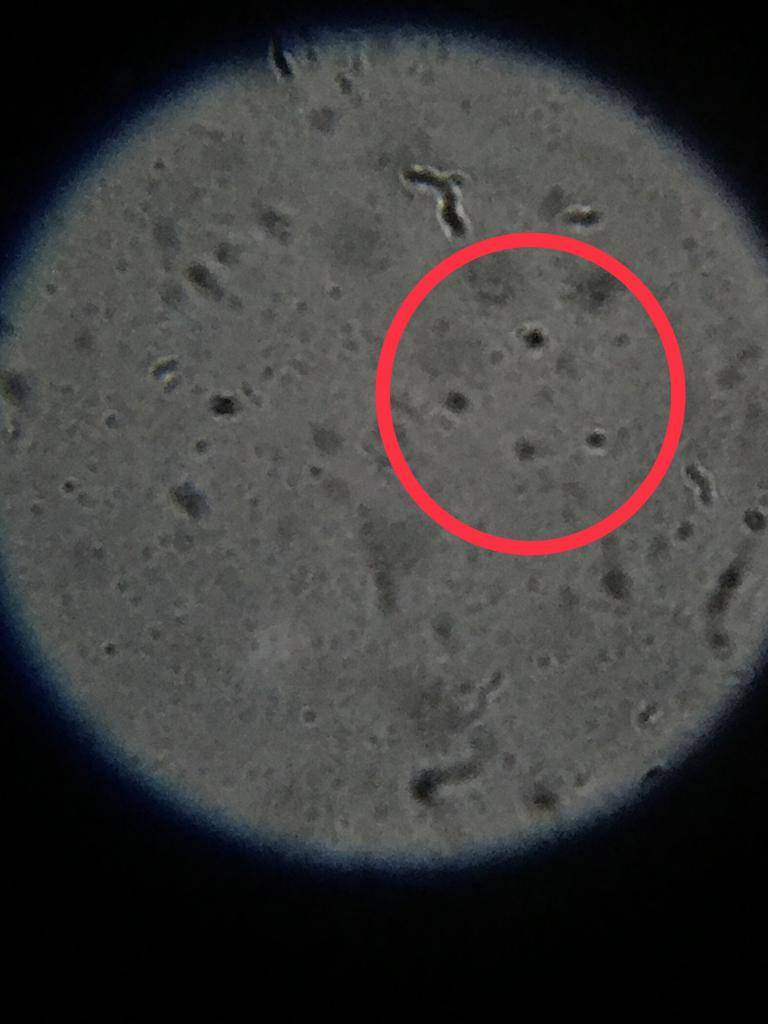
Rhodobacter?
I have a 10 second video of various creatures zipping around the field of view. I’ll try to find somewhere to upload it.
Success!
They say seeing is believing. Today I slipped into the lab over my lunch break to use the microscope. This scope is equipped with a camera, but the laptop that hooks up to it was being used. So I took the pictures using my cellphone over the eyepiece. I used the first successful bottle (white cap @ 28 days) for my sample.
Here’s what we’re looking for
Source: https://www.mbio.ncsu.edu/mb452/purple_nonsulfurs/purples.html
What did I find? A ton of life!
Rhodospirillum - Live
Rhodospirillum - Dead
Rhodospirillum is the easiest to identify because they’re large and have a unique shape. I’m hoping Microbeman can chime in and confirm/deny these others. Again, microscopy is not my forte.
Rhodomicrobium?
Rhodobacter?
I have a 10 second video of various creatures zipping around the field of view. I’ll try to find somewhere to upload it.


