Greenfox recently brought CAT Scientific's Debunking the Myth of THC-CBN Conversion Intensity study at https://www.catscientific.com/decarboxylating-cannabis/ to my attention, and asked my read, as it appeared to fly in the face of conventional protocol. My study at:
https://thealchemistresource.thealchemistresource.com/p/blog-page_10.html
Because I am familiar with the author, have tested the accuracy of the CAT Scientific hot plate, and know the Phd who tested the results, instead of simply shrugging it off, I dug deeper, starting with contacting Dr Justin Fischedick and seeking his insight on the phenomenon.
Always a delight, Dr Fischedick not only provided me with his thoughts, but provided me with three different serious scientific papers on the subject, which caused me to rewrite our own decarboxylation article and to add a couple more related threads to our TAR site.
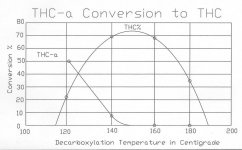
The conflict between the CAT Scientific study and conventional protocol, was the rate of THC conversion to CBN after reaching the peak of the 70% decarboxylation curve. It showed a slower drop in THC than the plunge suggested by the generally accepted graph on the subject, provided by the Journal of Chromatography in 1990 .
Graph 1
The conventional wisdom to this point is that once 70% decarboxylation is reached, that the rate the remaining THC-a is converted to THC is lower than the rate that the existing THC is converted to CBN.
That turned out to not be so, as demonstrated by this graph, which I drew using the copyrighted graph published in Isolation of D9-THC-a from hemp and analytical aspects concerning the determination of D9-THC in cannabis products, published in Forensic Science International, on line18-Aug-2004
Insert graph 2
As you can see, what this graph shows is that by the time we've reached 70% decarboxylation, 30% of the THC has already been turned to CBN and other breakdown products. The reason that THC doesn't continue to rise above about 70%, is there is no THC-a left to convert.
That still doesn't resolve the differences between the rate of THC conversion to CBN to the right of the graph, after it has peaked around 70%, shown here:
Insert graph 3
As you can see in the CAT Scientific study, the rate of THC decomposing into CBN is radically different than the plunge demonstrated in the previous graph.
The answers to that question lay in the different reports, as well as within the keen fine minds of Dr. Fishchedick.
The issue is that the tests and graphs don't represent the same test material or conditions.
The Journal of Chromatography graph represented a dab of concentrate heated in an open glass container, while the next graph was in a closed container excluding atmospheric oxygen, and the Cat Scientific study was done suspended in oil.
Dr. Fischedick relayed yet another test he had run, testing formulation stability, where they placed the THC + formula in an oven and flooded the oven with oxygen. Even in that oxygen enriched atmosphere, he said that the degradation was slower then the first two graphs above.
He also noted that many of his clients research producing CBN under various conditions and it varies for all of them until they really find an optimized process.
In addition we routinely inject THC into the inert atmosphere of a gas chromatographs at high temperatures like those shown and we don't see any degradation in that time period.
He summarized by saying that unless you're heating a couple grams of oil on a hot plot exactly like the first graph, we shouldn't expect conversion to be close to the same.
https://thealchemistresource.thealchemistresource.com/p/blog-page_10.html
Because I am familiar with the author, have tested the accuracy of the CAT Scientific hot plate, and know the Phd who tested the results, instead of simply shrugging it off, I dug deeper, starting with contacting Dr Justin Fischedick and seeking his insight on the phenomenon.
Always a delight, Dr Fischedick not only provided me with his thoughts, but provided me with three different serious scientific papers on the subject, which caused me to rewrite our own decarboxylation article and to add a couple more related threads to our TAR site.
The conflict between the CAT Scientific study and conventional protocol, was the rate of THC conversion to CBN after reaching the peak of the 70% decarboxylation curve. It showed a slower drop in THC than the plunge suggested by the generally accepted graph on the subject, provided by the Journal of Chromatography in 1990 .
Graph 1
The conventional wisdom to this point is that once 70% decarboxylation is reached, that the rate the remaining THC-a is converted to THC is lower than the rate that the existing THC is converted to CBN.
That turned out to not be so, as demonstrated by this graph, which I drew using the copyrighted graph published in Isolation of D9-THC-a from hemp and analytical aspects concerning the determination of D9-THC in cannabis products, published in Forensic Science International, on line18-Aug-2004
Insert graph 2
As you can see, what this graph shows is that by the time we've reached 70% decarboxylation, 30% of the THC has already been turned to CBN and other breakdown products. The reason that THC doesn't continue to rise above about 70%, is there is no THC-a left to convert.
That still doesn't resolve the differences between the rate of THC conversion to CBN to the right of the graph, after it has peaked around 70%, shown here:
Insert graph 3
As you can see in the CAT Scientific study, the rate of THC decomposing into CBN is radically different than the plunge demonstrated in the previous graph.
The answers to that question lay in the different reports, as well as within the keen fine minds of Dr. Fishchedick.
The issue is that the tests and graphs don't represent the same test material or conditions.
The Journal of Chromatography graph represented a dab of concentrate heated in an open glass container, while the next graph was in a closed container excluding atmospheric oxygen, and the Cat Scientific study was done suspended in oil.
Dr. Fischedick relayed yet another test he had run, testing formulation stability, where they placed the THC + formula in an oven and flooded the oven with oxygen. Even in that oxygen enriched atmosphere, he said that the degradation was slower then the first two graphs above.
He also noted that many of his clients research producing CBN under various conditions and it varies for all of them until they really find an optimized process.
In addition we routinely inject THC into the inert atmosphere of a gas chromatographs at high temperatures like those shown and we don't see any degradation in that time period.
He summarized by saying that unless you're heating a couple grams of oil on a hot plot exactly like the first graph, we shouldn't expect conversion to be close to the same.