This isa little guide to the pour through method for testing soil and soiless media for PH and EC(salinity) to make sure they are in correct levels or if there is a problem.
The Pour Thru Method for Testing Container Media
Steps for the Pour Thru method
1. water containers to saturation (so that a few drops of water come out of the bottom of the container) with the normal irrigation water they have been receiving
2. after container has drained for one hour, place a saucer under the container
3. pour enough distilled (DI) water on the surface of the container to get 50 mL (1.5 fluid ounces) of leachate to come out of the bottom of the container (Table 1)
4. collect leachate for pH and EC testing
5. calibrate pH and EC meters
6. measure pH and EC of samples
Saturated media extract (SME)
SME is currently "the" method of testing soilless greenhouse media and it is almost universally done by commercial and university labs, including the UMass Soil and Plant Tissue Testing Lab. In this test a paste is made using soil and water and then the liquid portion (the extract) is separated from the solid portion for pH, soluble salt, and nutrient analysis. Special skills and laboratory equipment are required to perform this test. SME is probably not suitable for a grower to use unless the greenhouse operation is large enough to support a lab, a technically trained person is hired to carry out the tests, and there is a commitment to frequent testing and tracking of the results.
1:2 dilution method
This method has been used for many years and has good interpretative data to back it up. In this test an air-dried sample of soil and water are mixed together in the volume ratio of 1 part soil to 2 parts water (e.g., using a measuring cup, 1 fl. oz. of soil + 2 fl. oz. of water). The liquid extract is then separated from the solids using laboratory grade filter paper or a common coffee filter. The extract is then ready for analysis. This is a very easy test to master and quite suitable for on-site greenhouse testing of pH and soluble salt using meters available from greenhouse suppliers. The 1:2 method is a very good choice for occasional pH and soluble salts testing by growers on-site.
INTERPRETING TEST DATA EC (Electrical Conductivity)
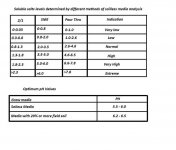
The values that you measure for EC will depend on the method you use for testing the container media. EC guidelines for several horticulture crops are presented in the table

Problems with Low EC
A low EC means that your plants are not getting enough fertilizer salts. Symptoms can include stunted plant growth or leaf discoloration due to lack of nutrients. Nitrogen deficiency (yellowing of lower leaves) often appears first.
Problems with High EC
Excess salts can accumulate when: you are applying more fertilizer than the plant requires; the container media has a high initial salt level; leaching during irrigation is insufficient; or your water source contains naturally high levels of salts. Excess salts can cause tissue death. Symptoms often appear first on the lower leaves and appear as yellowing (chlorosis) or browning (necrosis) that begins at the edges of the leaves and spreads inward. High salts can cause root tips to die back; and plants may show wilting even though the medium is still moist. High salt levels have been shown to increase the incidence of Pythium root rot. Solutions to high salts include leaching with clear water, then cutting back on the fertilizer rate if that was what caused salts to accumulate in the first place.
pH
pH affects the ability of nutrients to dissolve in water (solubility). Solubility is important because roots can only take up nutrients that are dissolved in solution and cannot take up the solid form of the nutrient.
Problems with Low pH
In container media, the micronutrients iron, manganese, zinc, and boron are highly soluble at low pH (pH 5.0-6.0). Therefore, at low pH these nutrients are available and readily taken up by roots. If pH is too low, typically below 5.0 for most plants, the nutrients become so soluble that they may be taken up at harmful or toxic concentrations. A classic symptom of this is iron toxicity which appears as leaf bronzing and chlorosis which appear first on lower leaves. Certain plants that are especially efficient at taking up iron, such as seed and zonal geraniums and marigolds, can exhibit iron toxicity when pH is below 6.0.
Problems with High pH
At high media pH the low solubility of phosphorus, iron, manganese, zinc, and boron (see figure below) makes these nutrients less available to be taken up by roots and so deficiency symptoms can occur. Certain plants are less efficient at absorbing micronutrients (especially iron and manganese). These plants require a slightly lower pH to be able to absorb enough of these nutrients. A classic example of this is iron deficiency is petunia. Affected plants show yellowing between the veins on the upper leaves. Often there is enough iron provided in the fertilizer/container media, but the pH is too high for roots to absorb it.
The Pour Thru Method for Testing Container Media
Steps for the Pour Thru method
1. water containers to saturation (so that a few drops of water come out of the bottom of the container) with the normal irrigation water they have been receiving
2. after container has drained for one hour, place a saucer under the container
3. pour enough distilled (DI) water on the surface of the container to get 50 mL (1.5 fluid ounces) of leachate to come out of the bottom of the container (Table 1)
4. collect leachate for pH and EC testing
5. calibrate pH and EC meters
6. measure pH and EC of samples
Saturated media extract (SME)
SME is currently "the" method of testing soilless greenhouse media and it is almost universally done by commercial and university labs, including the UMass Soil and Plant Tissue Testing Lab. In this test a paste is made using soil and water and then the liquid portion (the extract) is separated from the solid portion for pH, soluble salt, and nutrient analysis. Special skills and laboratory equipment are required to perform this test. SME is probably not suitable for a grower to use unless the greenhouse operation is large enough to support a lab, a technically trained person is hired to carry out the tests, and there is a commitment to frequent testing and tracking of the results.
1:2 dilution method
This method has been used for many years and has good interpretative data to back it up. In this test an air-dried sample of soil and water are mixed together in the volume ratio of 1 part soil to 2 parts water (e.g., using a measuring cup, 1 fl. oz. of soil + 2 fl. oz. of water). The liquid extract is then separated from the solids using laboratory grade filter paper or a common coffee filter. The extract is then ready for analysis. This is a very easy test to master and quite suitable for on-site greenhouse testing of pH and soluble salt using meters available from greenhouse suppliers. The 1:2 method is a very good choice for occasional pH and soluble salts testing by growers on-site.
INTERPRETING TEST DATA EC (Electrical Conductivity)
The values that you measure for EC will depend on the method you use for testing the container media. EC guidelines for several horticulture crops are presented in the table
Problems with Low EC
A low EC means that your plants are not getting enough fertilizer salts. Symptoms can include stunted plant growth or leaf discoloration due to lack of nutrients. Nitrogen deficiency (yellowing of lower leaves) often appears first.
Problems with High EC
Excess salts can accumulate when: you are applying more fertilizer than the plant requires; the container media has a high initial salt level; leaching during irrigation is insufficient; or your water source contains naturally high levels of salts. Excess salts can cause tissue death. Symptoms often appear first on the lower leaves and appear as yellowing (chlorosis) or browning (necrosis) that begins at the edges of the leaves and spreads inward. High salts can cause root tips to die back; and plants may show wilting even though the medium is still moist. High salt levels have been shown to increase the incidence of Pythium root rot. Solutions to high salts include leaching with clear water, then cutting back on the fertilizer rate if that was what caused salts to accumulate in the first place.
pH
pH affects the ability of nutrients to dissolve in water (solubility). Solubility is important because roots can only take up nutrients that are dissolved in solution and cannot take up the solid form of the nutrient.
Problems with Low pH
In container media, the micronutrients iron, manganese, zinc, and boron are highly soluble at low pH (pH 5.0-6.0). Therefore, at low pH these nutrients are available and readily taken up by roots. If pH is too low, typically below 5.0 for most plants, the nutrients become so soluble that they may be taken up at harmful or toxic concentrations. A classic symptom of this is iron toxicity which appears as leaf bronzing and chlorosis which appear first on lower leaves. Certain plants that are especially efficient at taking up iron, such as seed and zonal geraniums and marigolds, can exhibit iron toxicity when pH is below 6.0.
Problems with High pH
At high media pH the low solubility of phosphorus, iron, manganese, zinc, and boron (see figure below) makes these nutrients less available to be taken up by roots and so deficiency symptoms can occur. Certain plants are less efficient at absorbing micronutrients (especially iron and manganese). These plants require a slightly lower pH to be able to absorb enough of these nutrients. A classic example of this is iron deficiency is petunia. Affected plants show yellowing between the veins on the upper leaves. Often there is enough iron provided in the fertilizer/container media, but the pH is too high for roots to absorb it.
Attachments
Last edited by a moderator: