poindexterous
Member
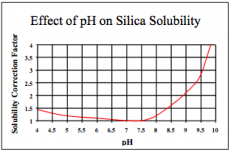
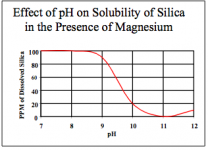
I'm sure I'm not the only one who sees a cloudiness form in the nutrient solution when adding high pH additives like pH raise or Pro-Tekt. It looks like underwater smoke. However, it quickly vanishes as soon as the high pH additive is diluted.
So the question I've had for years is: Is the cloudiness a permanent lockout of some element? Or is it just for the few seconds the cloudiness lasts and then it re-absorbs and all is fine? And what is the chemical reaction that causes it? Is it even a lockout at all?
Even diluting my pH raise to a fraction of full strength doesn't prevent it. Maybe 100 to 1 would, but 10 to 1 doesn't
There's certainly no warning on any pH raise I've seen, so you'd think it was not a problem. Dry pH raise like GH's is very strong, almost as alkaline as Crystal Draino, and they say to add it directly to the rez without diluting in water first.
Anyone know for sure?
Thanks!
P
So the question I've had for years is: Is the cloudiness a permanent lockout of some element? Or is it just for the few seconds the cloudiness lasts and then it re-absorbs and all is fine? And what is the chemical reaction that causes it? Is it even a lockout at all?
Even diluting my pH raise to a fraction of full strength doesn't prevent it. Maybe 100 to 1 would, but 10 to 1 doesn't
There's certainly no warning on any pH raise I've seen, so you'd think it was not a problem. Dry pH raise like GH's is very strong, almost as alkaline as Crystal Draino, and they say to add it directly to the rez without diluting in water first.
Anyone know for sure?
Thanks!
P