-
Happy Birthday ICMag! Been 20 years since Gypsy Nirvana created the forum! We are celebrating with a 4/20 Giveaway and by launching a new Patreon tier called "420club". You can read more here.
-
Important notice: ICMag's T.O.U. has been updated. Please review it here. For your convenience, it is also available in the main forum menu, under 'Quick Links"!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Absolute Amber from Banana Silver Ladyboys
- Thread starter jump117
- Start date
Jump117 asked me to repost this here in his glorious AA thread
I know a lot of people shy away from winterizing because they think using a second solvent with a much higher boiling point will kill all the flavor. But at full vacuum water will boil violently at room temp with no added heat. I don't have the exact temp that ethanols boiling point is lowered to under full vacuum at room temp, and will dig that up when I get some time. There is no need to use temps any higher than you would purging your bho under full vacuum and constant heat. It all depends on the viscosity of your amber, which usually depends on the age of the starting material.
I'll do some research and repost the azeotrope of 95% ethanol 5% water boiling point under full vacuum if any one else know's please post.
Absolutes ftw!
Check out jumps Absolute amber thread! https://www.icmag.com/ic/showthread.php?t=168388
The best way to hold onto the most flavor and terpenes when making an absolute is don't heat the ethanol to speed up the dissolving of the raw bho and there is no need to fully purge the initial butane extraction this will help you save the lighter terpenes. Purge residual ethanol in thin film under deep vacuum and constant heat of around 110-130F depending on the viscosity of the amber, there is no need to get your temps up to ethanols boiling point of 173F under vacuum because all boiling points are lowered under full vacuum.
I know a lot of people shy away from winterizing because they think using a second solvent with a much higher boiling point will kill all the flavor. But at full vacuum water will boil violently at room temp with no added heat. I don't have the exact temp that ethanols boiling point is lowered to under full vacuum at room temp, and will dig that up when I get some time. There is no need to use temps any higher than you would purging your bho under full vacuum and constant heat. It all depends on the viscosity of your amber, which usually depends on the age of the starting material.
I'll do some research and repost the azeotrope of 95% ethanol 5% water boiling point under full vacuum if any one else know's please post.
Absolutes ftw!

gunnaknow
Active member
I'll do some research and repost the azeotrope of 95% ethanol 5% water boiling point under full vacuum if any one else know's please post.
In a perfect vacuum, the boiling point becomes the same as the melting point. The boiling point of a substance is simply the point at which it's vapor pressure equals the surrounding pressure. If there is no surrounding pressure, no vapor pressure is needed for the substance to boil and it will therefore begin to boil at or above the melting point. This is assuming that the alcohol was already liquid before it was put under a perfect vacuum, as a solid cannot melt under a perfect vaccum and will simply sublimate. However, you can't create a perfect vacuum with a vacuum pump, so the substance will have to be slightly above it's melting point in order to boil. If you want to work out the boiling point within a partial vacuum then all you have to do is look at the vapor pressure curves for ethanol and water. For instance, the boiling point of ethanol at 40 mm hg is 19°C and for water it's 34°C. Taking into account the partial pressure of each, the boiling point of a 95% azeotrope would be 19.8°C. Although, strictly speaking the boiling point is for individual compounds, mixtures have a boiling range between their bubble point and dew point.
A short note on the azeotrope -
And let me repeat the question to the users of vacuum, please.
Is it possible to effectively increase the evaporation surface and the use volume of a vacuum chamber
by arranging several evaporation dishes, one over the other?
Quote from the thread "Thin film vacuum purging" -

In a vacuum azeotropic point shifts in favor of EtOH -
0.895 mol under 760 mm Hg ,
0.996 mol under 100 mm Hg .
And let me repeat the question to the users of vacuum, please.
Is it possible to effectively increase the evaporation surface and the use volume of a vacuum chamber
by arranging several evaporation dishes, one over the other?
Quote from the thread "Thin film vacuum purging" -
Thin layer is good for purging, but small in volume,
vacuum is running slow, so recharging is not often,
may be to increase productivity in the vacuum chamber can accommodate several floors with Teflon pans?

A short note on the azeotrope -
And let me repeat the question to the users of vacuum, please.
Is it possible to effectively increase the evaporation surface and the use volume of a vacuum chamber
by arranging several evaporation dishes, one over the other?
Quote from the thread "Thin film vacuum purging" -

Yes.
A short note on the azeotrope -
And let me repeat the question to the users of vacuum, please.
Is it possible to effectively increase the evaporation surface and the use volume of a vacuum chamber
by arranging several evaporation dishes, one over the other?
Quote from the thread "Thin film vacuum purging" -

I have done this before , you just have to be careful to leave enough space between the trays so the muffin doesn't hit the bottom of the one on top of it. It is also harder to keep the top trays the ideal temperature. I have had good success with two glass dishes, one on the bottom of the vac oven and the other on a stand above it. I think a really big shallow vac chamber or oven, with one thin layer only, would be the best choice for vac purging concentrates.
This^^^I have done this before , you just have to be careful to leave enough space between the trays so the muffin doesn't hit the bottom of the one on top of it. It is also harder to keep the top trays the ideal temperature. I have had good success with two glass dishes, one on the bottom of the vac oven and the other on a stand above it. I think a really big shallow vac chamber or oven, with one thin layer only, would be the best choice for vac purging concentrates.
Ya in a vacuum oven more then one dish would work fine, regular degassing chamber heated from below not so much. The bottom dish would be way overheated by the time the one above it got up to proper purging temps.
Perhaps you could have graced Jump with more than a one word answer, GW? It can sound a little blunt, even if it isn't intended that way.
Sorry, social graces are my least strong suite. Especially when tired and in pain.
Yes you can, but for the reasons HL outlined, it is less than optimal.
I haven't tried it in a vacuum oven, but it works for cold boiling in a vacuum chamber, though not for final finish, due to the lack of temperature control.
I also watch a vacuum purge closely to keep track of progress and it is not the same for all Petri dishes, if there is more than one. Other than checking it out, the first time the subject came up, I haven't repeated it.
gunnaknow
Active member
Total______5,45 g ____100%
Cake______4,25
decarbed___0,15______3%
filter’______0,05______ 1%
second____ 0,4 _______7%
first_______0,6 ______11%
------------------------------
resin______ 1,2 g ____22%
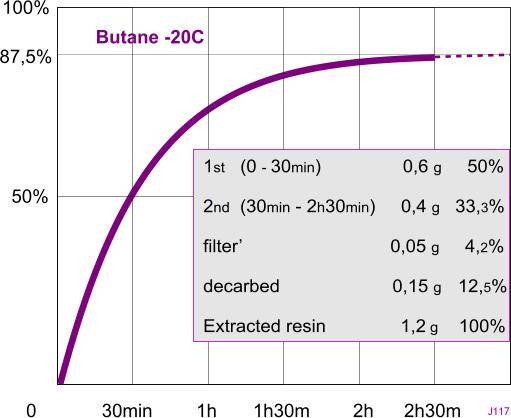
Another excellent experiment, jump. The main reason why I began advocating the use of a thermos, was to maximize the extraction time and the return. Your experiment helps to show others the evidence behind the theory. Apart from my old post in 2006, I haven't been inclined toward demonstrating to others, so it really helps that you're willing to do this for others.
gunnaknow
Active member
Cheers mate. I'd like to think that I've contributed a few ideas to the community but so have many others, including yourself. Your experiments have demonstrated to others the practicalities of such methods and you've added many new ideas to the mix. It's a collaborative effort, which everyone can get involved in. I'm looking forward to your next installment of beautifully sculpted goodies. Take care.
gunna
gunna
Why is this glorious thread no longer a sticky?
Bobbito Blaze
Member
Instead of the ethanol, did you or do you know if you can use toluene?



