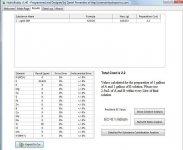
I believe that the 140g you are referring to is to make a liter. Liter bottles are easy to come by. Just get a bottle of soda and you have one. The 530g was to make a gallon at 98ppm though. 541g will do a gallon at 100ppm. The numbers come directly from the link were Spurr said to buy it at.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
How to make silicone and MagiCal?
- Thread starter sexybudandnugs
- Start date
Wish I could find hydrobuddy on my computer. I've downloaded it every time I have used it, such a pain in my uglier side. I pulled my numbers from the suppliers site.
sexybudandnugs
Member
If you take that Epson salt and add it to the calcium nitrate you will get a precipitate of calcium sulphate. You need a magnesium without the sulfur.
I was going to use CaCl2 not Ca(NO3)2
Does that matter? Will it form insoluble salts?
sexybudandnugs
Member
OK so I found a post by the Technaflora rep on this site.
http://www.technaflora.com/phpBB2/viewtopic.php?t=488&sid=85c59b67f283d546fd341f8c2adb22a3
Here he states that Magical has:
N = 30 ppm
Ca = 42
Mg = 16
for 5ml per gallon.
So the above info is incorrect. This will also lower the chlorine which is a good thing.
So the correct info to make 1 liter will be this:
Magnesium nitrate 128g
calcium nitrate 56g
calcium chloride 66g
iron DTPA Not sure on this one as I didn't get the ppm or %
If some one has the iron numbers we can post how much iron DTPA to add.
Why can't you just use CaCl2? Why must we use calcium nitrate as well? Where would you find this locally? I bet it's more money. I can get almost pure CaCl2 for $25 for about 50 lbs.
I don't care about the Iron (Fe), I bet my nutes have that. All these Micronutrients I have covered or at least look it so I don't think it is a big deal to have the iron.
Using you numbers x 3.785 to get one gallon
MgNO32 - 484.48 g
CaNO32 - 212 g
CaCl2 - 249.81 g
These large numbers were very similar to my large numbers... of course we are using different salts... So I believe my formulation was pretty accurate as well... of course I'm not sure if CaCl2 and MgSO4 ONLY is okay to use. Many people use this formulation in salt water fish tanks. But stored in two different containers.
http://reefkeeping.com/issues/2006-02/rhf/index.php
How did you know this new formula would have less chloride?
sexybudandnugs
Member
Let's check your numbers as well:
MgNO32 - 484.48 g
CaNO32 - 212 g
CaCl2 - 249.81 g
Periodic Table:
Mg = 24.3 g, NO3 = 62g (2NO3 = 124 g)
MgNO32 = 148.3 g
24.3 / 148.3 = 16.4% of Mg
484.48 x .164 = 79.4 g of Mg
79.4 g / 3785 g = 2% Mg
Using that formulation looks like 2% Mg.
MagiCal says, 1.25% Mg
http://www.technaflora.com/indexProduct.php?ID=103
MgNO32 - 484.48 g
CaNO32 - 212 g
CaCl2 - 249.81 g
Periodic Table:
Mg = 24.3 g, NO3 = 62g (2NO3 = 124 g)
MgNO32 = 148.3 g
24.3 / 148.3 = 16.4% of Mg
484.48 x .164 = 79.4 g of Mg
79.4 g / 3785 g = 2% Mg
Using that formulation looks like 2% Mg.
MagiCal says, 1.25% Mg
http://www.technaflora.com/indexProduct.php?ID=103
sexybudandnugs
Member
Ca = 40.1 , Cl2 = 71, CaCl2 = 111.1
40.1/111.1 = x/249.81
x = 90.2 g of Ca
CaNO32 = 40.1 + 124 = 164.1
40.1/164.1 = x/212
x = 51.8 g of Ca
142g of Ca / 3785 g = 3.75% of Ca
MagiCal says, 3.25% Ca
http://www.technaflora.com/indexProduct.php?ID=103
That is a good recipe.
I think my calculation is a little different because I am doing a 1 gallon total (add salt where you add to one gallon of water total). But since there is so much salt (solute) the final weight won't really be 3785 g (the weight of 1 gallon of water) which usually doesn't matter with a little solute.
BTW, Botanicare Cal-Mag Plus, which I used before MagiCal (both work) doesn't even use CaCl2 just CaNO32
http://www.planetnatural.com/site/cal-mag.html
40.1/111.1 = x/249.81
x = 90.2 g of Ca
CaNO32 = 40.1 + 124 = 164.1
40.1/164.1 = x/212
x = 51.8 g of Ca
142g of Ca / 3785 g = 3.75% of Ca
MagiCal says, 3.25% Ca
http://www.technaflora.com/indexProduct.php?ID=103
That is a good recipe.
I think my calculation is a little different because I am doing a 1 gallon total (add salt where you add to one gallon of water total). But since there is so much salt (solute) the final weight won't really be 3785 g (the weight of 1 gallon of water) which usually doesn't matter with a little solute.
BTW, Botanicare Cal-Mag Plus, which I used before MagiCal (both work) doesn't even use CaCl2 just CaNO32
http://www.planetnatural.com/site/cal-mag.html
You can use calcium chloride if you want it just adds chloride to the water. If you use magnesium sulphate you just can't mix it ahead of time with the calcium or it will precipitate. At what concentration I'm not sure.
I get my calcium nitrate locally for $19 at the farm store Hamilton's. That is for 50 pounds.
When you go here
http://agr.wa.gov/PestFert/Fertilizers/FertDB/prodinfo.aspx?pname=4942
it shows no chlorine in the Magical. Since they only have to report a minimum amount in the product I think they are adding a little more calcium nitrate to their mix and just reporting less nitrate in their product. If you want to use the chloride it is your choice as I don't know what it will do to your plants.
How I knew it would add less chloride was that the magnesium amount went down and when that goes down so does the amount of nitrate from it. This left more nitrate for the calcium nitrate which allows for more calcium nitrate.
If you go to the custom hydr nutrients site they list How many ppm per unit of measure for the ingredients they sell. I did not break every thing down by molar weight.
here is the site:
http://customhydronutrients.com/zencart/
All my calculations were based on ppm not % as that is what I was going off. I was not going off of the bottles I was going off the Techniflora reported ppm. So this could be some of our differences.
I get my calcium nitrate locally for $19 at the farm store Hamilton's. That is for 50 pounds.
When you go here
http://agr.wa.gov/PestFert/Fertilizers/FertDB/prodinfo.aspx?pname=4942
it shows no chlorine in the Magical. Since they only have to report a minimum amount in the product I think they are adding a little more calcium nitrate to their mix and just reporting less nitrate in their product. If you want to use the chloride it is your choice as I don't know what it will do to your plants.
How I knew it would add less chloride was that the magnesium amount went down and when that goes down so does the amount of nitrate from it. This left more nitrate for the calcium nitrate which allows for more calcium nitrate.
If you go to the custom hydr nutrients site they list How many ppm per unit of measure for the ingredients they sell. I did not break every thing down by molar weight.
here is the site:
http://customhydronutrients.com/zencart/
All my calculations were based on ppm not % as that is what I was going off. I was not going off of the bottles I was going off the Techniflora reported ppm. So this could be some of our differences.
sexybudandnugs
Member
You can use calcium chloride if you want it just adds chloride to the water. If you use magnesium sulphate you just can't mix it ahead of time with the calcium or it will precipitate. At what concentration I'm not sure.
I get my calcium nitrate locally for $19 at the farm store Hamilton's. That is for 50 pounds.
When you go here
http://agr.wa.gov/PestFert/Fertilizers/FertDB/prodinfo.aspx?pname=4942
it shows no chlorine in the Magical. Since they only have to report a minimum amount in the product I think they are adding a little more calcium nitrate to their mix and just reporting less nitrate in their product. If you want to use the chloride it is your choice as I don't know what it will do to your plants.
How I knew it would add less chloride was that the magnesium amount went down and when that goes down so does the amount of nitrate from it. This left more nitrate for the calcium nitrate which allows for more calcium nitrate.
If you go to the custom hydr nutrients site they list How many ppm per unit of measure for the ingredients they sell. I did not break every thing down by molar weight.
here is the site:
http://customhydronutrients.com/zencart/
All my calculations were based on ppm not % as that is what I was going off. I was not going off of the bottles I was going off the Techniflora reported ppm. So this could be some of our differences.
Ichabod, you're right CaSO4 is not to soluble in water... I considered that to be insoluble or slight soluble which sucks!
Solubility in water:
0.21g/100ml at 20°C (anhydrous)
0.24 g/100ml at 20°C (dihydrate)
Surprisingly, it actually gets less soluble as temp goes up!
So I could make two separate solutions but what's the point? I think I might find some MgCl2, I already have CaCl2. These salts come so pure for so cheap. If not I'll but CaNO32 and MgNO32. Better off if I find the it locally like you that would kick ass! But how do you get the MgNO32 is there a way to get that locally?
Nice find on the product information page but how do you know that is MagiCal? It says "PRODUCT: HAY MAKER 18-3-4"
Do you mean http://agr.wa.gov/PestFert/Fertilizers/FertDB/prodinfo.aspx?pname=4950
I think the Chloride should be safe especially in those small amounts as a supplement. They even use it on salt water fish tanks which is WAY more sensitive. Table salt has chloride in it (sodium chloride).
Thanks for the info!
sexybudandnugs
Member
Yes, but that gives you nothing of worth wrt ppm of Si. And the 'ppm' from a so-called "PPM Meter" is not the same as true ppm (elemental) found via math. All a so-called PPM Meter does is make a stupid conversion from EC. You should use an EC meter, not a PPM meter.
So I got around to measure the ppm with my ppm meter. I can't measure MagiCal and Pro-Tek in their regular state because it is too concentrated, even though my ppm meter goes to the thousands. I used R/O D/I water for all purposes consider it distilled water. It has 0 ppm.
1 tsp of MagiCal in 1 gallon of water:
202 ppm (197-202 ppm)
1 tsp of Pro-Tek in 1 gallon water:
57-58 ppm
Anyone do it with AgSil16 or their bootleg MagiCal?
sexybudandnugs
Member
Originally Posted by sexybudandnugs
Wouldn't ~90 ppm Si just be double the formula ? 1.4 grams in one gallon of water?
Quote from Spurr:
No, not exactly. My thread is on the first page of this sub-forum, here:
https://www.icmag.com/ic/showthread.php?t=222646
See reading that makes me thing Spurr said you can't just double up the AgSil and assume it has twice as much Si... but I'll look into it.
Wouldn't ~90 ppm Si just be double the formula ? 1.4 grams in one gallon of water?
Quote from Spurr:
No, not exactly. My thread is on the first page of this sub-forum, here:
https://www.icmag.com/ic/showthread.php?t=222646
See reading that makes me thing Spurr said you can't just double up the AgSil and assume it has twice as much Si... but I'll look into it.
Sorry that is the wrong link . on this page you can find all of Washington states listed fertilizers.
http://agr.wa.gov/PestFert/Fertilizers/ProductDatabase.aspx
Here is the correct link for magical.
http://agr.wa.gov/PestFert/Fertilizers/FertDB/prodinfo.aspx?pname=4950
As far a doubling the agsil you do not need to as that is what protekt has is the lower number. I think you are confusing Si with SiO3.
As for the Ca maybe you could use calcium carbonate to get the Ca in solution. This is not very soluble at higher PH but is at a lower PH. If I am not mistaken calcium carbonate is just quicklime. So maybe your concentrate could have a lower PH until it is diluted into your final solution. You could use phosphoric acid or HCL to get this PH. I would stay away from sulfuric because you do not want to form calcium sulphate.
http://agr.wa.gov/PestFert/Fertilizers/ProductDatabase.aspx
Here is the correct link for magical.
http://agr.wa.gov/PestFert/Fertilizers/FertDB/prodinfo.aspx?pname=4950
As far a doubling the agsil you do not need to as that is what protekt has is the lower number. I think you are confusing Si with SiO3.
As for the Ca maybe you could use calcium carbonate to get the Ca in solution. This is not very soluble at higher PH but is at a lower PH. If I am not mistaken calcium carbonate is just quicklime. So maybe your concentrate could have a lower PH until it is diluted into your final solution. You could use phosphoric acid or HCL to get this PH. I would stay away from sulfuric because you do not want to form calcium sulphate.
sexybudandnugs
Member
Yea CaCO3 would be cool to use to get the Ca in. People in salt water use baking soda (also known as Sodium bicarbonate or sodium hydrogen carbonate). It's food grade and very soluble. If you heat it in the over on 400 degrees it's actually better (totally NOT necessary though). It is used for stabilize pH and to raise alkalinity. Where can you buy CaCo3? I'm going to catch up on Spurr's videos and stuff...
"Spread baking soda (594 grams or about 2 ¼ cups) on a baking tray and heat in an ordinary oven at 300°F for one hour to drive off water and carbon dioxide. This solution will contain about 1,900 meq/L of alkalinity (5,300 dKH). I prefer to use baked baking soda rather than washing soda in this recipe as baking soda from a grocery store is always food grade, while washing soda may not have the same purity requirements. Arm & Hammer brand is a fine choice. Be sure to NOT use baking powder. Baking powder is a different material that often has phosphate as a main ingredient."
Actually that might be good for us.... phosphate... hmmm...... right?
"Spread baking soda (594 grams or about 2 ¼ cups) on a baking tray and heat in an ordinary oven at 300°F for one hour to drive off water and carbon dioxide. This solution will contain about 1,900 meq/L of alkalinity (5,300 dKH). I prefer to use baked baking soda rather than washing soda in this recipe as baking soda from a grocery store is always food grade, while washing soda may not have the same purity requirements. Arm & Hammer brand is a fine choice. Be sure to NOT use baking powder. Baking powder is a different material that often has phosphate as a main ingredient."
Actually that might be good for us.... phosphate... hmmm...... right?
You should be able to get it at a pool supply company, or at a building supply company that works with concrete or brick. CaCO3 is used to stabilize pool PH. It is also used in cement.
sexybudandnugs
Member
To get it more like Pro-Tek, which is about a 2:1 weight ratio (SiO2:K2O, 7.8%:3.7%), wouldn't AgSil 21 be a better choice over AgSil 16H? The downside is that AgSil 21 has less total weight for SiO2 (26.5%) as to AgSil 16H (52.8%). You will use A LOT more of this product. The upside is that it will be more like Pro-Tek. I don't think it is worth the trade off because K2O isn't as important as the SiO2 we are trying to obtain. However, here is my calculations to get 100 ppm using AgSil 20. Based on their website (http://customhydronutrients.com/zencart/index.php?main_page=product_info&cPath=36_74&products_id=122) AgSil 20 is 12.7% K2O 26.5% SiO2 2.1 weight ratio (SiO2:K2O).
Therefore, if you want to use 100 pm of SiO2 using AgSil20:
C (ppm) = mass of solute (mg) / V of solution (l) , since solution is water, mass of 1 kg is about 1 liter.
100 = x/3.785
x = 378.5 mg
So we need 378.5 mg of SiO2 but remember it is only 26.5% of the weight:
.265y=378.5 mg
y = 1,428 mg or 1.448 grams of AgSil 20 will give about 100 ppm of SiO2.
Remember with AgSil 16H it was only .7 g to get 98 ppm of SiO2. This makes sense since AgSil is 26.5% SiO2 and AgSil 16H is 52.8% which is double the percentage! So you will need double the amount!
*Once again, I don't recommend it. But that's the calculations! They don't have the calculations for ppm on the website like they have for AgSil 16H.
Therefore, if you want to use 100 pm of SiO2 using AgSil20:
C (ppm) = mass of solute (mg) / V of solution (l) , since solution is water, mass of 1 kg is about 1 liter.
100 = x/3.785
x = 378.5 mg
So we need 378.5 mg of SiO2 but remember it is only 26.5% of the weight:
.265y=378.5 mg
y = 1,428 mg or 1.448 grams of AgSil 20 will give about 100 ppm of SiO2.
Remember with AgSil 16H it was only .7 g to get 98 ppm of SiO2. This makes sense since AgSil is 26.5% SiO2 and AgSil 16H is 52.8% which is double the percentage! So you will need double the amount!
*Once again, I don't recommend it. But that's the calculations! They don't have the calculations for ppm on the website like they have for AgSil 16H.
sexybudandnugs
Member
The best I can do on this is this. The Pro-tekt says on the back of the bottle that 5ml will give you 100ppm per gallon of silicate, which is SiO2. So the web site pointed out by Spurr says to add .7g to one gallon to get 98 ppm per gallon of solution. If this is the case take a one liter bottle and add 140g of potassium silicate (.7g times 200)(1 liter equals 5ml X 200) and then top off with RO water. This should be close to the Pro-Tekt.
A gallon has 3785ml per gallon. So that means 757 5ml doses per gallon. So to make a gallon of Pro-Tekt you need to mix 530g of potassium silicate to a gallon jug and then top off with RO water.
Your cost then is about $3.7 per liter plus shipping.
Not saying this is exact but I hope it helps.
I have the bottle of Pro-Tek in front on me. It says this.
"1 tsp. Pro-Tekt/gal=100 ppm of silicon"
I agree 1 tsp and 5 ml are NEARLY equivalent (~4.93). However, is silicate and silicone interchangeable? Are both referring to SiO2? BTW, if you use 4.93 ml instead of 5 ml, you get 142 g per liter and 537.4 g per gallon.
Also to get 100 ml instead of 98 ppm you need .71428 g instead of .7 g, using ~4.93 ml instead of 5 ml, it is 142.86 g per liter or 540.7 g per gallon.
sexybudandnugs
Member
Some more calculations to check the numbers on custom hydroponics:
YaraLiva CalcInit, "19% calcium. One gram dissolved in one gallon of water yeilds 41 ppm nitrate nitrogen and 50 ppm calcium."
C (ppm) = mass of solute (mg) / V of solution (l) , since solution is water, mass of 1 kg is about 1 liter.
(1 g x 19%) is .19 g of calcium
ppm = 190 milligrams of Ca / (1kg x 3.785)
50.19 ppm of Ca
Similarly, 15.5 g of Nitrate
.155 g of Nitrate
ppm = 155 milligrams of Nitrate/ (1 kg x 3.785)
40.95 ppm of Nitrate
The numbers have been check!
YaraLiva CalcInit, "19% calcium. One gram dissolved in one gallon of water yeilds 41 ppm nitrate nitrogen and 50 ppm calcium."
C (ppm) = mass of solute (mg) / V of solution (l) , since solution is water, mass of 1 kg is about 1 liter.
(1 g x 19%) is .19 g of calcium
ppm = 190 milligrams of Ca / (1kg x 3.785)
50.19 ppm of Ca
Similarly, 15.5 g of Nitrate
.155 g of Nitrate
ppm = 155 milligrams of Nitrate/ (1 kg x 3.785)
40.95 ppm of Nitrate
The numbers have been check!
sexybudandnugs
Member
From Custom on Magnisal:
With 11% Nitrate nitrogen and 9.6% magnesium. One gram in one gallon of water yeilds 29ppm Nitrate nitrogen and 25ppm Magnesium.
C (ppm) = mass of solute (mg) / V of solution (l) , since solution is water, mass of 1 kg is about 1 liter.
1 g of Magnisal is (9.6% x 1) = .096 g of Mg
ppm = 96 milligrams of mg / (1kg x 3.785)
25.36 ppm of Mg.
With 11% Nitrate nitrogen and 9.6% magnesium. One gram in one gallon of water yeilds 29ppm Nitrate nitrogen and 25ppm Magnesium.
C (ppm) = mass of solute (mg) / V of solution (l) , since solution is water, mass of 1 kg is about 1 liter.
1 g of Magnisal is (9.6% x 1) = .096 g of Mg
ppm = 96 milligrams of mg / (1kg x 3.785)
25.36 ppm of Mg.
sexybudandnugs
Member
Using CaCl2 (Dow) instead of CaNO32 is better in the sense that you will get more Calcium per weight. So basically you have to use less salt. Which saves you money. Also, you can find locally easily (home improvement store). CaNO32 is better so you get nitrates as well. I think most nutes have enough nitrates, correct me if I am wrong. I don't see why Cl would be bad for the reservoir either.
The Dow calcium chloride I use is typically 94% (94%-97%)! http://anchsand.com/Portals/3/calciumchloride_product.pdf
The Dow Custom offers is great, however it is 83%-87% (mostly just water difference).
Typical is 85% !! http://www.liquidcalciumchloride.com/Literature/ProductInfo/DOWFLAKEProdInfo.pdf
Custom uses 87% in it's calculations (best case scenario)
http://customhydronutrients.com/zencart/index.php?main_page=product_info&cPath=1_47_86&products_id=140
Using the 87% (I think they should use 85% which is typical) they got
"83-87% pure Calcium Chloride Dowflake xtra. At 87% pure Dowflake is 31.68% Calcium and 55.68% Chlorine. One gram in one gallon of water yeilds 84ppm Calcium and 147ppm Chlorine."
Let's check their numbers:
C (ppm) = mass of solute (mg) / V of solution (l) , since solution is water, mass of 1 kg is about 1 liter.
1 g of CaCl2 is (.87 x 1 x 36%[using periodic table Ca is 36% of the weight of CaCl2]) = .3131 g of Ca (or more accurately, at most, .314157, hmm...looks like Pi... any relation? Anyway, that's 31.42% not 31.68% like Custom says)
ppm = 313.1 milligrams of Ca / (1kg x 3.785)
82.7 ppm of Ca.
(I believe Custom is wrong for the first time!)
Not only that... TYPICALLY it is 85% NOT 87%.... Let me do the calculation for you for 85%:
C (ppm) = mass of solute (mg) / V of solution (l) , since solution is water, mass of 1 kg is about 1 liter.
1 g of CaCl2 is (.85 x 1 x 36%[using periodic table Ca is 36% of the weight of CaCl2]) = .306 g of Ca (Anyway, that's 30.6% not 31.68% like Custom says)
ppm = 306 milligrams of Ca / (1kg x 3.785)
80.8 ppm of Ca.
Using that salt will give 81 ppm NOT 84 ppm. Correct me if I am wrong!
I have Typical 94% CaCl2, here are the calculations for that:
C (ppm) = mass of solute (mg) / V of solution (l) , since solution is water, mass of 1 kg is about 1 liter.
1 g of CaCl2 is (.94 x 1 x 36%[using periodic table Ca is 36% of the weight of CaCl2]) = .3384 g of Ca
ppm = 338.4 milligrams of Ca / (1kg x 3.785)
89.4 ppm of Ca.
I think I might use CaCl2 instead of CaNO32, unless someone tells me other wise. I'm going to look for CaNO32 though.
I'll check out the MagiCal formula you made Icha! Good nite!
The Dow calcium chloride I use is typically 94% (94%-97%)! http://anchsand.com/Portals/3/calciumchloride_product.pdf
The Dow Custom offers is great, however it is 83%-87% (mostly just water difference).
Typical is 85% !! http://www.liquidcalciumchloride.com/Literature/ProductInfo/DOWFLAKEProdInfo.pdf
Custom uses 87% in it's calculations (best case scenario)
http://customhydronutrients.com/zencart/index.php?main_page=product_info&cPath=1_47_86&products_id=140
Using the 87% (I think they should use 85% which is typical) they got
"83-87% pure Calcium Chloride Dowflake xtra. At 87% pure Dowflake is 31.68% Calcium and 55.68% Chlorine. One gram in one gallon of water yeilds 84ppm Calcium and 147ppm Chlorine."
Let's check their numbers:
C (ppm) = mass of solute (mg) / V of solution (l) , since solution is water, mass of 1 kg is about 1 liter.
1 g of CaCl2 is (.87 x 1 x 36%[using periodic table Ca is 36% of the weight of CaCl2]) = .3131 g of Ca (or more accurately, at most, .314157, hmm...looks like Pi... any relation? Anyway, that's 31.42% not 31.68% like Custom says)
ppm = 313.1 milligrams of Ca / (1kg x 3.785)
82.7 ppm of Ca.
(I believe Custom is wrong for the first time!)
Not only that... TYPICALLY it is 85% NOT 87%.... Let me do the calculation for you for 85%:
C (ppm) = mass of solute (mg) / V of solution (l) , since solution is water, mass of 1 kg is about 1 liter.
1 g of CaCl2 is (.85 x 1 x 36%[using periodic table Ca is 36% of the weight of CaCl2]) = .306 g of Ca (Anyway, that's 30.6% not 31.68% like Custom says)
ppm = 306 milligrams of Ca / (1kg x 3.785)
80.8 ppm of Ca.
Using that salt will give 81 ppm NOT 84 ppm. Correct me if I am wrong!
I have Typical 94% CaCl2, here are the calculations for that:
C (ppm) = mass of solute (mg) / V of solution (l) , since solution is water, mass of 1 kg is about 1 liter.
1 g of CaCl2 is (.94 x 1 x 36%[using periodic table Ca is 36% of the weight of CaCl2]) = .3384 g of Ca
ppm = 338.4 milligrams of Ca / (1kg x 3.785)
89.4 ppm of Ca.
I think I might use CaCl2 instead of CaNO32, unless someone tells me other wise. I'm going to look for CaNO32 though.
I'll check out the MagiCal formula you made Icha! Good nite!
have the book:
Gardening Indoors with Soil and Hydroponics by George F Van Patten.
In this book it states "Chlorine is vital to increase the cellular osmotic pressure, modify the stomata regulation, and augment the plant's tissue and moisture content. A solution concentration of 140 parts per million (ppm) is usually safe for plants, but some varieties may show sensitivity when foliage turns pale green and wilts. Excess chlorine causes the leaf tips and margins to burn and causes the leaf to turn a bronze color."
Thought this would help you if you are going to use chloride in your mix. Another thing you may try is to use pool shock and bubble the chlorine out over night, but not sure if it could be made into a concentrate this way.
Gardening Indoors with Soil and Hydroponics by George F Van Patten.
In this book it states "Chlorine is vital to increase the cellular osmotic pressure, modify the stomata regulation, and augment the plant's tissue and moisture content. A solution concentration of 140 parts per million (ppm) is usually safe for plants, but some varieties may show sensitivity when foliage turns pale green and wilts. Excess chlorine causes the leaf tips and margins to burn and causes the leaf to turn a bronze color."
Thought this would help you if you are going to use chloride in your mix. Another thing you may try is to use pool shock and bubble the chlorine out over night, but not sure if it could be made into a concentrate this way.