Hi all
Been busy these times with extractions...
One of the method i really enjoyed is BHO.
I used Colibri gas as it's the easiest to find zero-impurities brand here in europe.
Amazing results...but still a lot of questions in my head...
Searching trough the net i've found that Colibri gas is a mixture, and has a Benchmark for nonvolatile compounds of 50ppm
Y'all know that these compounds are dangerous for health.
I was trying to find some purer Gases and i found that the purest is "Research-grade purity" at 99.910% N-Butane gas. This is Not a mixture of different gases, just butane in its normal form.
These are the specs:
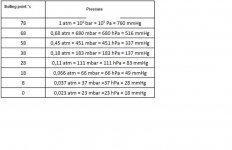
(BP means Boiling point at sea level)
[FONT=Times New Roman,Times,serif]1,3-Butadiene < 1 ppm BP: -4.4°c / 24F
1-Butene < 1 ppm BP: -6,3°c / 21F
2-Butene (cis) < 5 ppm BP: 4°c
2-Butene (trans) < 5 ppm BP: 1°c
2,2-Dimethylpropane < 2 ppm BP: 9.5°c
Ethane < 1 ppm BP: -89°c /
Ethylene < 1 ppm BP: -103.7°C / -154.7F
Isobutane < 500 ppm BP: -11.7°C / 11F
Isobutylene < 100 ppm BP: -6.9°c / 20F
Isopentane < 2 ppm BP:[/FONT] 27.7°c
[FONT=Times New Roman,Times,serif] Methane < 1 ppm BP: -161.6°c / -259F
Nitrogen < 5 ppm BP: -195.8°c / -320F
Oxygen < 5 ppm BP: -182.9°c / 297F
Pentane < 2 ppm BP: 36.1°c
Propane < 300 ppm BP: -42°c / -44F
Propylene < 2 ppm BP: -47°c / -54F
Water < 1 ppm[/FONT] BP: 100°c
The remaing part should be n-butane with a BP of -0.5°c
Since i can't find a clear definition of what is a Non-volatile compund, i've underlined in red the substances with higher BP, so they are more difficult to get rid during the purge.
Which is the real minimum evaporation temp. of the Butane+resin mix?
On my hand it is at leats 40°c.
I've found that if i spray on a surface some butane gas it will evap in seconds at room temp, BUT if i extract some resin, the liquid resulting won't evap so easily as only butane do.
My questions are:
Is this Research grade butane suitable for extractions?
Is it anyhow safer than normal colibri gas?
is it worth try to find this purer gas?
[FONT=Times New Roman,Times,serif][/FONT]
Been busy these times with extractions...
One of the method i really enjoyed is BHO.
I used Colibri gas as it's the easiest to find zero-impurities brand here in europe.
Amazing results...but still a lot of questions in my head...
Searching trough the net i've found that Colibri gas is a mixture, and has a Benchmark for nonvolatile compounds of 50ppm
Y'all know that these compounds are dangerous for health.
I was trying to find some purer Gases and i found that the purest is "Research-grade purity" at 99.910% N-Butane gas. This is Not a mixture of different gases, just butane in its normal form.
These are the specs:
(BP means Boiling point at sea level)
[FONT=Times New Roman,Times,serif]1,3-Butadiene < 1 ppm BP: -4.4°c / 24F
1-Butene < 1 ppm BP: -6,3°c / 21F
2-Butene (cis) < 5 ppm BP: 4°c
2-Butene (trans) < 5 ppm BP: 1°c
2,2-Dimethylpropane < 2 ppm BP: 9.5°c
Ethane < 1 ppm BP: -89°c /
Ethylene < 1 ppm BP: -103.7°C / -154.7F
Isobutane < 500 ppm BP: -11.7°C / 11F
Isobutylene < 100 ppm BP: -6.9°c / 20F
Isopentane < 2 ppm BP:[/FONT] 27.7°c
[FONT=Times New Roman,Times,serif] Methane < 1 ppm BP: -161.6°c / -259F
Nitrogen < 5 ppm BP: -195.8°c / -320F
Oxygen < 5 ppm BP: -182.9°c / 297F
Pentane < 2 ppm BP: 36.1°c
Propane < 300 ppm BP: -42°c / -44F
Propylene < 2 ppm BP: -47°c / -54F
Water < 1 ppm[/FONT] BP: 100°c
The remaing part should be n-butane with a BP of -0.5°c
Since i can't find a clear definition of what is a Non-volatile compund, i've underlined in red the substances with higher BP, so they are more difficult to get rid during the purge.
Which is the real minimum evaporation temp. of the Butane+resin mix?
On my hand it is at leats 40°c.
I've found that if i spray on a surface some butane gas it will evap in seconds at room temp, BUT if i extract some resin, the liquid resulting won't evap so easily as only butane do.
My questions are:
Is this Research grade butane suitable for extractions?
Is it anyhow safer than normal colibri gas?
is it worth try to find this purer gas?
[FONT=Times New Roman,Times,serif][/FONT]