St. Phatty
Active member
I have been thinking about Bottle Rockets.

On some of the Space Shuttle launches, they use a Solid Fuel Rocket, like a bottle rocket.
Those 2 white things on the side - special solid fuel rockets designed for Space Shuttle launches.
https://en.wikipedia.org/wiki/Space_...lid_Rocket_Boo ster
2.8 Million Pounds of Thrust from each booster. They used the Deion Sanders approach and used 2.
When I was younger, we lived in Greece, and it was my turn to have a Lesser Bedroom. I slept in a hall closet next to my older brother's bedroom.
My father was a chemist, and he told my older brother what to buy for fireworks.
Of course, the factory shops in Athens were plenty happy to sell Potassium Perchlorate to a 16 year old American kid.
So he made fireworks in his bedroom, next to my Hall closet.
So I was about 14, and my next younger brother was about 11.
The younger brother made a mistake lighting somebody else's home-made firework.
The fuse burned too fast, and gave him a serious burn on his hand. Fortunately it didn't explode.
So I look at these larger rockets, and I wonder - what do the Space Shuttle astronauts think about sitting on top of 2 boosters, each with 1.1 Million pounds of Solid Rocket fuel ?
Isn't it kind of important that it burn slowly, and not all at once ?
"Ammonium perchlorate composite propellant (APCP) is a modern fuel used in solid-propellant rocket vehicles. It differs from many traditional solid rocket propellants such as black powder or zinc-sulfur, not only in chemical composition and overall performance but also by the nature of how it is processed. APCP is cast into shape, as opposed to powder pressing as with black powder."
How do you cast 1.1 million pounds of APCP ? Besides, very carefully.
When they cast the APCP into shape, Do they just turn the rocket fuel chamber upside down, and pour in the fuel after it's been heated enough to make it pour-able ? Sounds like they make a bunch of sections. 22 sections of fuel each weighing 50,000 pounds might be easier to handle than 1 big section with 1.1 Million pounds of fuel ?
ORGANIC ROCKET FUEL
Sometimes, people use Black Powder as a rocket propellant.
Potassium Nitrate, Carbon, and Sulfur.
Contains 3 of the 6 primary & secondary plant fertilizers !
"Black powder (gunpowder) propellant[edit]
Black powder (gunpowder) is composed of charcoal (fuel), potassium nitrate (oxidizer), and sulfur (fuel and catalyst). It is one of the oldest pyrotechnic compositions with application to rocketry. In modern times, black powder finds use in low-power model rockets (such as Estes and Quest rockets),[SUP][23][/SUP][SUP][24][/SUP] as it is cheap and fairly easy to produce."

Good Pot today !
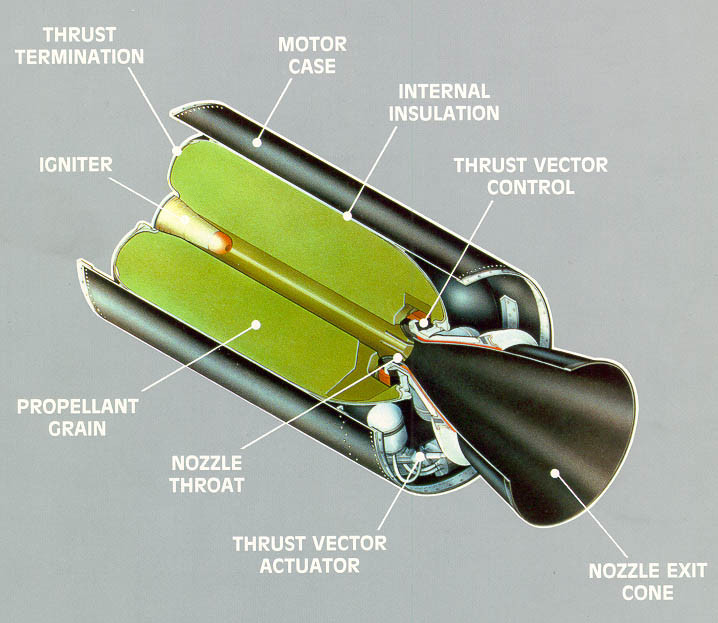

On some of the Space Shuttle launches, they use a Solid Fuel Rocket, like a bottle rocket.
Those 2 white things on the side - special solid fuel rockets designed for Space Shuttle launches.
https://en.wikipedia.org/wiki/Space_...lid_Rocket_Boo ster
2.8 Million Pounds of Thrust from each booster. They used the Deion Sanders approach and used 2.
When I was younger, we lived in Greece, and it was my turn to have a Lesser Bedroom. I slept in a hall closet next to my older brother's bedroom.
My father was a chemist, and he told my older brother what to buy for fireworks.
Of course, the factory shops in Athens were plenty happy to sell Potassium Perchlorate to a 16 year old American kid.
So he made fireworks in his bedroom, next to my Hall closet.
So I was about 14, and my next younger brother was about 11.
The younger brother made a mistake lighting somebody else's home-made firework.
The fuse burned too fast, and gave him a serious burn on his hand. Fortunately it didn't explode.
So I look at these larger rockets, and I wonder - what do the Space Shuttle astronauts think about sitting on top of 2 boosters, each with 1.1 Million pounds of Solid Rocket fuel ?
Isn't it kind of important that it burn slowly, and not all at once ?
"Ammonium perchlorate composite propellant (APCP) is a modern fuel used in solid-propellant rocket vehicles. It differs from many traditional solid rocket propellants such as black powder or zinc-sulfur, not only in chemical composition and overall performance but also by the nature of how it is processed. APCP is cast into shape, as opposed to powder pressing as with black powder."
How do you cast 1.1 million pounds of APCP ? Besides, very carefully.
When they cast the APCP into shape, Do they just turn the rocket fuel chamber upside down, and pour in the fuel after it's been heated enough to make it pour-able ? Sounds like they make a bunch of sections. 22 sections of fuel each weighing 50,000 pounds might be easier to handle than 1 big section with 1.1 Million pounds of fuel ?
ORGANIC ROCKET FUEL
Sometimes, people use Black Powder as a rocket propellant.
Potassium Nitrate, Carbon, and Sulfur.
Contains 3 of the 6 primary & secondary plant fertilizers !
"Black powder (gunpowder) propellant[edit]
Black powder (gunpowder) is composed of charcoal (fuel), potassium nitrate (oxidizer), and sulfur (fuel and catalyst). It is one of the oldest pyrotechnic compositions with application to rocketry. In modern times, black powder finds use in low-power model rockets (such as Estes and Quest rockets),[SUP][23][/SUP][SUP][24][/SUP] as it is cheap and fairly easy to produce."

Good Pot today !

